Abstract
Background
Treatment options for melanoma in situ (MIS) include imiquimod, radiation therapy, cryotherapy, excisional and Mohs surgery. Ingenol mebutate is a new topical treatment option recognized for actinic keratosis. Although in vitro effectiveness has been demonstrated on melanoma cell lines, its therapeutic potential for in vivo melanomas is unknown.
Case Report
In 2011, a 91-year-old woman presented a thick melanoma of her cheek. The lateral sections revealed persisting in situ melanoma, which were again excised. She presented for follow-up and a recurrent MIS was evidenced centered on the previous scar. She refused further surgery and ingenol mebutate (0.015% gel) was administered on three consecutive days. One month later, a complete clinical resolution was observed. Histology and immunohistology revealed no residual MIS.
Conclusion
In this patient, ingenol mebutate was successful and well-tolerated as a topical, alternative therapy for MIS after failure of other treatment options.
Similar content being viewed by others
Introduction
Ingenol mebutate (a macrocyclic diterpene ester, PEP005, 0.015%) derives from the sap of the plant Euphorbia peplus. This herb has been used of old as a traditional remedy for skin cancers. Ingenol mebutate is currently an US Food and Drug Administration (FDA) and European Medicines Agency (EMA) recognized field-directed treatment for actinic keratosis [1, 2]. Ingenol mebutate activates a broad range of protein kinase C (PKC) (α, β, γ, δ, ε, η and θ) isoenzymes [3]. Direct pro-apoptotic effects of this drug have been demonstrated in several malignant cells, including melanoma cell lines. Micromolar concentrations of ingenol mebutate are required to kill melanoma cells via PKC-independent secondary necrosis [3]. Topical application of ingenol mebutate was revealed as being effective for human and murine melanoma in mouse models [3]. In transformed keratinocytes, ingenol mebutate leads to cell death. Furthermore, ingenol mebutate promotes an inflammatory infiltrate that kills remaining tumor cells. It has been demonstrated that ingenol mebutate does not mediate cytotoxicity by a simple lytic, necrotic mechanism, but activates distinct processes involving multiple cell organelles in a cell-type and differentiation-dependent manner [4]. In vitro experiments revealed epidermal cell death, acute inflammation, recruitment of neutrophils, hemorrhage, and eschar formation when ingenol mebutate was tested on keratinocytic cell lines and squamous cell cancer cell lines [5, 6]. Currently, the action mechanism of ingenol mebutate is divided into three consecutive steps; (1) a direct effect of the drug on the initial cancer accompanied by an in situ production of pro-inflammatory cytokines, (2) neutrophil infiltration, and (3) induction of tumor-reactive antibodies, diminishing potential relapses by antibody-dependent neutrophil cytotoxicity [3].
However, as far as the authors are aware, no published data are available on a potential effect of ingenol mebutate on in vivo melanoma.
Melanoma in situ (MIS) is the most superficial form of melanoma [7, 8]. There are different treatment options including topical immunotherapy by imiquimod, topical destructive treatment by cryotherapy, superficial radiotherapy and excisional or Mohs surgery, all presenting their respective advantages and inconveniencies. Some patients do not qualify anymore for surgery for various reasons and topical treatments are then preferred.
This case report describes an elderly woman with two previous large excisions of thick melanoma on her cheek who refused further surgery for recurrent MIS on the surgical scars.
Case Report
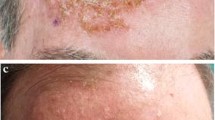
A 91-year-old woman presented with 2 biopsy-proven recurrent MISs in the direct vicinity of a scar on her right cheek (Fig. 1). In 2011, she was diagnosed with a superficial melanoma of the right cheek (0.14 mm depth, Clark II, KI67: 10%, 1 mitosis/mm2, with micro metastases) arising from an MIS and a nodular melanoma of the right cheek (4 mm depth, Clark V, KI67: 30%, >5 mitoses/mm2) (Fig. 2). A wide surgical excision was performed respecting 2 cm surgical margins. The patient refused further workup for staging and was followed in the dermatology department. One year later, she presented a recurrent nodular melanoma. Again, wide surgery was performed. Histology revealed a nodular melanoma (1, 93 mm depth, Clark IV, KI67: 30%, 3 mitoses/mm2, with micro metastases). Further physical examination was unremarkable. Due to her age and her own wishes, no further staging examinations were performed. Two years later, she presented with 2 recurrent MISs adjacent to the surgical scar (Fig. 2). A 4-mm punch biopsy confirmed the diagnosis of MIS of the lentigo maligna melanoma type. Again the patient and her family refused categorically any further surgical intervention and the authors decided to attempt to treat these lesions with ingenol mebutate gel 0.015%, based on in vitro data on melanoma cell lines [3]. Three consecutive daily applications were performed on the lesion area. On the day of the third application, a moderately crusting and oozing reaction was observed. According to the severity scale assessing ingenol mebutate toxicity, the composite score was 9/24 (erythema: 1, flaking or scaling: 2, crusting: 4, swelling: 2, vesiculation or postulation: 0 and erosion or ulceration: 0) [9]. Topical disinfection and topical antibiotic ointment were recommended and the crust disappeared after 1 week. One month later, there was a clinical resolution of both lesions, with a slightly squamous, post-inflammatory erythema (Fig. 3). A cutaneous biopsy proved the absence of residual MIS on histology (Fig. 4) and on using immunohistochemistry with NKI-C3, S100a, HMB45 and Melan A (DAKO, Glostrup, Denmark) (Fig. 5). The local tolerance of the treatment was acceptable for the patient and no systemic signs were observed. After 6 months of follow-up the patient was still free of MIS recurrence.
Discussion
Standard care for MIS is surgical excision or Mohs surgery respecting a margin of 5–9 mm of clinically non-involved healthy skin [7]. However, alternative treatment options for MIS are sometimes required for different reasons. Either the patient refuses excisional surgery or surgery is no longer recommended due to morbidity and/or cosmetic considerations [8]. Furthermore, older age, multiple previous surgical procedures, medical comorbidities may preclude from surgery. The non-invasive treatment options for MIS include local radiation therapy and topical immunotherapy. Occasionally cryotherapy or cryosurgery could also be advocated.
Radiation therapy uses either Grenz rays or soft X-rays. Although published cure rates achieving 86% to 95%, it remains an uncommonly used treatment option for MIS in poor candidates for surgery.
Imiquimod is a synthetic imidazoquinoline amine and acts as a toll-like receptor (TLR) agonist on TLR7 and TLR8 [7–11]. This topical immunomodulator increases, after binding to the TLR’s, the endogenous production of interferon-alpha, exhibiting anticancer properties, interleukins (ILs) 6 and 12, as well as tumor necrosis factor alpha [7, 8]. Evidence-based medicine data on imiquimod and MIS are scarce. Most publications deal with small case series and case reports [6]. A recent meta-analysis showed an average clearance rate of 91% with a histological proof [7]. However, the follow-up periods were usually short, the “negative biopsy” did not preclude persisting areas of MIS and particular attention should be given to invasive melanoma progression after an initial superficial tumor clearing.
There is no standard recommendation on dosing and application protocols of imiquimod and adaptations are often required to minimize the local adverse reactions, consisting of moderate to severe erythema, crusting, and sometimes oozing, usually occurring after 2–3 weeks of treatment. Systemic effects may sometimes be observed in the form of flu-like symptoms with fever, headache, hyperesthesia, myalgia and fatigue.
In sum, the long-term cure rates of imiquimod are questionable [7] and caution is advocated not to fail a subsequent diagnosis of residual or recurrent invasive melanoma [7].
This case report suggests that in some selected patients with MIS, ingenol mebutate could be considered as an alternative treatment. It could present the advantage over imiquimod not to display potential systemic flu-like symptoms. Furthermore, although cutaneous adverse effects are common for both agents, they are usually shorter in duration with ingenol mebutate.
Dosing regimens, treatment efficacy and tolerance should be evaluated on larger series. Further research on the “cytotoxicity/inflammation” mechanisms of action of ingenol mebutate on in vivo melanocytic tumors is warranted.
Conclusion
Ingenol mebutate merits to be considered as an alternative treatment option for MIS in selected patients, after other therapies have failed. Patients should be followed closely to detect eventual recurrent deep invasion of melanoma.
References
Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013; 149:666–70.
Keating GM. Ingenol mebutate gel 0.015% and 0.05%: in actinic keratosis. Drugs. 2012;72:2397–405.
Ersvaer E, Olsnes Kittang A, Hampson P, et al. The protein kinase C agonist PEP005 (Ingenol 3-Angelate) in the treatment of human cancer: a balance between efficacy and toxicity. Toxins. 2010; 2:174-94.
Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181–92.
Cozzi SJ, Le TT, Ogbourne SM, James C, Suhrbier A. Effective treatment of squamous cell carcinomas with ingenol mebutate gel in immunologically intact SKH1 mice. Arch Dermatol Res. 2013;305:79–83.
Cozzi SJ, Ogbourne SM, James C, et al. Ingenol mebutate field-directed treatment of UVB-damaged skin reduces lesion formation and removes mutant p53 patches. J Invest Dermatol. 2012;132:1263–71.
Erickson C, Miller SJ. Treatment options in melanoma in situ: topical and radiation therapy, excision and Mohs surgery. Int J Dermatol. 2010;49:482–91.
Toren KL, Parlette EC. Managing melanoma in situ. Semin Cutan Med Surg. 2010;29:258–63.
Lebwohl M1, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010–9.
Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107–8.
Ellis LZ, Cohen JL, High W, Stewart L. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937–46.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article.
All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
M. Mansuy, N. Nikkels-Tassoudji, J.E. Arrese, Andree Rorive and Arjen F. Nikkels declare no conflicts of interest.
Compliance with ethics
Informed consent was obtained from the patient for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mansuy, M., Nikkels-Tassoudji, N., Arrese, J.E. et al. Recurrent In Situ Melanoma Successfully Treated with Ingenol Mebutate. Dermatol Ther (Heidelb) 4, 131–135 (2014). https://doi.org/10.1007/s13555-014-0051-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-014-0051-4