Abstract
Introduction
Inadequately controlled asthma is associated with increased healthcare resource utilization. The eXpeRience registry was initiated to evaluate real-world outcomes in patients receiving omalizumab for uncontrolled persistent allergic asthma. The current analysis of data from the eXpeRience registry focuses on healthcare resource utilization and on absences from work or school.
Methods
The eXpeRience was a 2-year, multinational, non-interventional, observational registry conducted to investigate real-world outcomes among patients receiving omalizumab in accordance with country-specific prescribing criteria for the treatment of uncontrolled persistent allergic asthma. Asthma-related healthcare resource utilization (hospitalizations, emergency room visits or unscheduled-asthma-related doctor visits or interventions) and absences from work or school were assessed pre-treatment (12-month data were collected retrospectively at baseline) and at months 12 and 24 after the initiation of omalizumab treatment. Serious adverse event (SAE) data were also assessed.
Results
A total of 943 patients (mean age 45 years; female 65%) were enrolled in the registry. Overall, the mean (standard deviation [SD]) number of asthma-related medical healthcare uses per patient decreased from 6.2 (6.97) during the pre-treatment period to 1.0 (1.96) and 0.5 (1.28) at months 12 and 24, respectively. The mean (SD) number of work or school days missed due to asthma was also lower at months 12 (3.5 [17.28] and 1.6 [4.28], respectively) and 24 (1.0 [4.66] and 1.9 [5.46], respectively) compared with the pre-treatment period (26.4 [49.61] and 20.7 [27.49], respectively). The nature and frequency of SAEs in the eXpeRience registry were comparable to that seen in interventional clinical trials with omalizumab.
Conclusion
The results of the eXpeRience registry indicate that omalizumab is associated with reductions in healthcare utilization, and in the number of days of absence from work or school, in patients with uncontrolled persistent allergic asthma in the real-world setting.
Funding
Novartis Pharma AG, Basel, Switzerland.
Similar content being viewed by others
Introduction
Asthma affects approximately, 300 million individuals worldwide and places substantial social and economic burdens on society [1, 2]. In the US, asthma was responsible for 456,000 hospitalizations, 1.75 million emergency room (ER) visits and 13.9 million physician visits annually between 2005 and 2009. In the same period, patients with asthma missed 14.2 million work days and 10.5 million school days per year [3]. Despite long-term treatment with inhaled corticosteroids (ICS) and long-acting β2-agonists (LABA), asthma remains poorly controlled in a significant proportion of patients [4]. Although poor compliance and incorrect inhaler technique explains inadequate disease control in some patients, many others remain uncontrolled despite correction of these factors [5].
Poorly controlled asthma doubles the mean annual total asthma costs based on direct (medication usage, unscheduled office visits, ER visits, hospitalizations) and indirect (school/work days lost) assessments, compared with asthma that is partially or well-controlled [6]. The rates of hospitalizations, ER visits and number of work/school days lost due to asthma can be significantly reduced by employing effective treatment strategies that improve disease management [3].
Omalizumab is a humanized anti-immunoglobulin E (IgE) monoclonal antibody that is approved for the treatment of patients with uncontrolled moderate-to-severe (US) or severe (EU) persistent allergic (IgE-mediated) asthma [7, 8]. The efficacy and safety of omalizumab in patients with moderate and severe allergic asthma have been demonstrated in several randomized clinical trials [9–13] and real-world clinical practice setting studies [14–18]. These studies have shown that adjunctive therapy with omalizumab significantly reduces asthma exacerbations and improves asthma control, lung function and asthma-related quality of life, compared with placebo [9–13]. However, data on healthcare resource utilization in patients with asthma are also needed to provide information on the overall burden of the disease, including cost [19].
Although several controlled clinical trials have demonstrated that omalizumab reduced the frequency of ER visits and asthma-related hospitalizations [11, 20, 21], there is limited corroborative evidence from real-world clinical practice. The eXpeRience registry was initiated to evaluate real-world outcomes in patients receiving omalizumab for uncontrolled persistent allergic asthma.
The primary results from eXpeRience have been published previously [22], and indicate that omalizumab is associated with improvements in clinical outcomes such as exacerbation rates and objective measures of asthma control. The current analysis of data from the eXpeRience registry focuses on healthcare resource utilization and on absences from work or school.
Methods
eXpeRience was a 2-year, multinational, non-interventional, observational registry established to collect data on the effectiveness and safety of omalizumab therapy, prescribed during routine clinical practice, in patients with uncontrolled persistent allergic asthma.
The registry design has been described previously [23]. Briefly, patients with uncontrolled persistent allergic asthma were recruited from 14 countries in Europe, North America and Asia. Patients were required to meet the local labeling requirements for omalizumab use, and to have commenced omalizumab within 15 weeks prior to inclusion in the registry. Patients who had received omalizumab within the previous 18 months, or who were concurrently enrolled in a clinical trial for asthma medication, were excluded.
At the time of a patient’s entry into the registry, medical records were used to provide information on endpoints of interest during the year before omalizumab initiation. After entry into the registry, data were collected prospectively at ~16 weeks and again at 8, 12, 18 and 24 months after initiation of omalizumab treatment, with a minimum requirement of two data collections per year. Annualized data were calculated for month 12 (combining week 16, month 8 and month 12 time-points) and month 24 (combining month 18 and month 24 time-points). Treatment and follow-up of patients was at the discretion of treating physicians, who followed local medical practice and labelling/reimbursement guidelines.
In accordance with local regulations, the registry design and amendments were reviewed by independent ethics committees or institutional review boards at each participating center. All procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the registry.
Assessments
Asthma-related healthcare resource utilization and absences from work or school were assessed pre-treatment (for the 12 months prior to omalizumab treatment) and at months 12 and 24 after the initiation of omalizumab. The outcome measures evaluated were: asthma-related hospitalizations, ER visits and unscheduled doctor visits; total medical healthcare use (a composite of asthma-related hospitalizations, ER visits and unscheduled doctor visits); and number of missed work or school days due to asthma.
Safety was assessed by recording the nature and frequency of serious adverse events (SAEs) that occurred during the registry.
Statistical Analysis
Data were analysed by Parexel International GmbH (Berlin, Germany), in accordance with the planned analysis. No formal statistical hypotheses were tested; statistical analyses were mainly descriptive. Disease background and demographic variables were summarized using mean and standard deviation (SD), and frequencies for discrete variables. Descriptive summaries are presented for healthcare resource utilization.
Results
Patient Disposition and Baseline Characteristics
A total of 943 patients were included in the registry. Of these patients, 694 (73.6%) completed the registry and 157 (16.6%) discontinued; status was unknown for 92 (9.8%). Patients discontinued from the registry for a variety of reasons, including: 52 (5.5%) patients were lost to follow-up; 27 (2.9%) patients withdrew their consent; 19 (2.0%) patients stopped treatment due to a lack of efficacy; 11 (1.2%) patients were non-compliant or stopped treatment without reason; 9 (1.0%) patients died during the registry timeline; 9 (1.0%) patients stopped treatment for pregnancy-related reasons; 9 (1.0%) patients stopped treatment due to adverse events; 7 (0.7%) patients moved away from the area during the registry timeframe; 6 (0.6%) stopped treatment due to financial reasons; 1 (0.1%) patient discontinued the registry at month 18 with the primary reason for discontinuation being 2 years follow-up period completed before month 24; 1 (0.1%) patient’s discontinuation reason was missing; and 6 (0.6%) patients discontinued treatment for other reasons not specified.
Baseline characteristics have been described previously [22]. In summary, patients had a mean age of 45 years, were predominantly female (65%), and had a median duration of asthma of 16 years.
The intent-to-treat population (patients who received at least one dose of omalizumab and had at least one post-baseline efficacy assessment) comprised 916/943 (97.1%) patients; 27 patients were excluded due to lack of post-baseline assessments. The safety population (those who had received at least one dose of omalizumab and had at least one post-baseline safety assessment) comprised 925/943 (98.1%) patients.
Effect of Omalizumab on Asthma-related Healthcare Resource Use
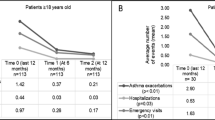
Annualized asthma-related hospitalizations, ER visits and unscheduled doctor visits were lower with omalizumab treatment at months 12 and 24 than during the pre-treatment period (Fig. 1). In addition, the proportion of patients with no annualized hospitalizations (Fig. 2a), ER visits (Fig. 2b) or unscheduled doctor visits (Fig. 2c) was higher at months 12 and 24 compared with the pre-treatment period.
Mean number of hospitalizations, ER visits and unscheduled doctor visits per patient. Annualized data are presented for month 12 (combining data from week 16, month 8 and month 12 time-points) and month 24 (combining data from the month 18 and month 24 time-points). * Within 12 months prior to the start of omalizumab treatment. Error bars represent standard deviation. ER emergency room
(a) Asthma-related hospitalizations (excluding patients with unknown status [pre-treatment n = 34; month 12 n = 32; month 24 n = 15]), (b) ER visits (excluding patients with unknown status [pre-treatment n = 49 month 12 n = 34; month 24 n = 16]), and (c) unscheduled doctor visits (excluding patients with unknown status [pre-treatment n = 93; month 12 n = 50; month 24 n = 24]). Annualized data are presented for month 12 (combining data from week 16, month 8 and month 12 time-points) and month 24 (combining data from the month 18 and month 24 time-points). *Within 12 months prior to the start of omalizumab treatment. ER emergency room
Overall, the mean (SD) total number of annualized asthma-related medical healthcare uses (hospitalizations, ER visits and unscheduled doctor visits) per patient decreased from 6.2 (6.97) during the pre-treatment period to 1.0 (1.96) and 0.5 (1.28) at months 12 and 24, respectively. The proportion of patients with no annualized asthma-related medical healthcare use increased from 12.3% (113/916) during the pre-treatment period to 60.2% (442/734) at month 12 and 75.4% (485/643) at month 24.
Effect of Omalizumab on Work/School Absence
The mean number of missed work or school days due to asthma was lower at months 12 and 24 than during the pre-treatment period (Fig. 3). The proportion of patients who did not miss any work days due to asthma increased from 16.4% (91/554) in the pre-treatment period to 57.6% (234/406) and 71.1% (256/360) at months 12 and 24, respectively. Similarly, the proportion of patients who did not miss any school days due to asthma was 13.1% (16/122) in the pre-treatment period, 66.7% (50/75) at month 12, and 64.0% (48/75) at month 24.
Mean number of work or school days missed per patient. Annualized data are presented for month 12 (combining data from week 16, month 8 and month 12 time-points) and month 24 (combining data from the month 18 and month 24 time-points). * Within 12 months prior to the start of omalizumab treatment. Error bars represent standard deviation. ER emergency room
Safety
Safety data have been described previously [22]. Briefly, 64 patients (6.9%) reported a total of 150 SAEs, with 28 reporting more than one SAE. The most common SAE was asthma (32 patients [3.5%]), followed by dyspnoea and pneumonia (each in 7 patients [0.8%]). Nine deaths occurred during the registry, none of which were suspected to be related to omalizumab.
Discussion
The results of this analysis of eXpeRience registry data suggest that add-on treatment with omalizumab is associated with reduced asthma-related hospitalizations, ER visits and unscheduled doctor visits in patients with uncontrolled persistent allergic asthma. It also demonstrated that the mean number of missed work or school days due to asthma decreased over 2 years of treatment with omalizumab compared with the pre-treatment period.
Asthma remains poorly controlled in many patients, leading to substantial financial burden in the developed world [24]. Inadequately controlled asthma is associated with increased utilization of healthcare resources, incurring a variety of direct (hospitalizations, ER visits, physician/specialist consultations, medication and laboratory tests) and indirect (e.g. absence from work or school) costs [4, 25]. In Europe, the total annual cost of asthma has been estimated at ~€17.7 billion, of which ~€9.8 billion is the indirect cost of lost productivity due to absence from work [26]. Management of persistent asthma has been estimated to incur mean annual costs of €1,583 per patient, 37.5% of which are direct (>50% for pharmacological treatment and 25% for hospital services) and 62.5% of which are indirect (lost working days or days with limited productivity) [27]. In addition, productivity losses due to asthma appear to be strongly associated with disease severity [28].
Due to the high socioeconomic burden and costs associated with healthcare resource use among patients with uncontrolled asthma [29], there is a need for interventions that have the potential to prevent exacerbations and reduce unscheduled healthcare contact. The results of this analysis of eXpeRience registry data are consistent with findings reported in clinical trials [11, 13], observational studies [14, 16–18, 30, 31] and in analyses of healthcare insurance claims [19]. In pivotal studies of omalizumab [9, 10, 12, 13], over 1,700 patients with moderate-to-severe allergic asthma were treated with omalizumab for 28–32 weeks. Taken as a whole, these studies showed that omalizumab-treated patients had significantly fewer hospitalizations, ER visits and unscheduled doctor visits than those receiving placebo [9, 10, 12, 13]. Furthermore, data from several observational studies [14, 17, 32] also corroborate the findings of the current registry. These observational studies, which included over 450 patients who received omalizumab for up to 52 weeks, explored the effectiveness of omalizumab as an add-on therapy. In the PERSIST study [14], 58.7% patients had fewer healthcare visits (a composite of general practitioner visits, specialist visits, ER visits and hospitalizations) at the end of the treatment period compared with the previous year. In two other studies [17, 32], annual hospitalization rates were found to be 29–96% lower, and ER visits 65–87% lower, after treatment with omalizumab. Additionally, in a retrospective analysis of health insurance claims, there were relative reductions of 40.8 and 48.6% in the rates of hospitalizations and ER visits, respectively, between the pre- and post-omalizumab treatment periods in patients with uncontrolled asthma [19]. This analysis also demonstrated significant reductions (p < 0.0001) in the mean number of ER visits and hospitalizations after initiation of omalizumab [19].
Similarly, several studies investigated the effect of omalizumab on the absence from work or school due to asthma [30, 33, 34]. In one of these studies, work or school days missed due to asthma was markedly reduced by 72.7 and 76.7% in patients aged ≥50 and <50 years, respectively, after 4 months of omalizumab treatment (p < 0.001) [33]. Treatment with omalizumab also led to a significant decrease in the mean number of work days lost in a study by Costello and colleagues [30]. In a double-blind randomized controlled study in children there was a significantly lower mean number of absences from school in children receiving omalizumab, in comparison with placebo, over the entire treatment period (p = 0.04) [34]. The results of the current, real-world analysis confirm and extend these findings, as over a 2-year period, the mean number of missed work or school days due to asthma decreased with omalizumab treatment compared with the pre-treatment period.
Data from clinical trials [11, 20, 35] and previously reported results from the eXpeRience registry [22] have shown that omalizumab is associated with reductions in exacerbations and with improvements in asthma control and symptoms. Marked reductions in oral corticosteroid use in patients receiving omalizumab have also been observed [22]. Asthma exacerbations are indicative of poor disease control [36]. Since uncontrolled asthma is associated with more exacerbations and healthcare visits versus controlled asthma [6, 29, 37, 38], a reduction in exacerbations would be expected to lead to a reduction in hospitalizations, ER visits and unscheduled doctor visits while simultaneously improving health-related quality of life [29, 39]. Similarly, better asthma control and reduced symptoms might be expected to improve attendance at school or work [1].
Although lack of efficacy was seen in 19 patients, in the worst case scenario, even if “non-compliant/stopping treatment without reason” and “AE related” can be considered as lack of efficacy, this percentage of patients is still relatively low (total n = 39; 4.1%).
Assuming the number of hospitalizations for those 39 patients at month 12 (in addition to 702 patients analysed) remained same as the average number of hospitalizations at pre-treatment visit (0.7, Fig. 1), the number of hospitalizations at month 12 would only increase by 0.04 per patient per annum. This increment would not influence the overall annualized hospitalization reduction effect of omalizumab in the registry population.
We therefore propose that the effects on healthcare resource use and on absence from work or school observed in the eXpeRience registry might reasonably be attributed to the positive impact of omalizumab treatment on exacerbations and asthma control. However, we also recognize that caution is warranted in interpreting the results from the eXpeRience registry, given its open-label, observational design; our findings may be partly attributable to factors other than the treatment of interest. Other possible limitations include patient selection criteria and the retrospective nature of pre-enrolment data collection.
Conclusions
To our knowledge, eXpeRience is the largest multinational study of real-world outcomes in patients receiving omalizumab for the treatment of allergic asthma. With the caveats noted above, we conclude that, in the real-world setting, treatment with omalizumab is associated with reductions in healthcare resource utilization and absence from work or school when added to current therapy in patients with uncontrolled persistent allergic asthma. The findings from the registry supplement the findings from the omalizumab clinical trial program and are likely to be relevant to the impact of omalizumab on the direct and indirect costs of healthcare and reducing socioeconomic burden of patients with uncontrolled allergic asthma.
References
Global Initiative for Asthma (2014) Global strategy for asthma management and prevention. http://www.ginasthma.org/documents/4. Accessed 20 Oct 2014.
To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204.
Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;32:1–14.
Chapman KR, Boulet LP, Rea RM, Franssen E. Suboptimal asthma control: prevalence, detection and consequences in general practice. Eur Respir J. 2008;31:320–5.
Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246–51.
Szefler SJ, Zeiger RS, Haselkorn T, et al. Economic burden of impairment in children with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2011;107:110–9.
EMA Xolair SmPC (2013). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000606/WC500057298.pdf. Accessed 20 Oct 2014.
US Food and Drug Administration (2014) Omalizumab (marketed as Xolair) Information. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm103291.htm. Accessed 20 Oct 2014.
Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–90.
Holgate ST, Chuchalin AG, Hébert J, et al. Omalizumab 011 international study group. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004;34:632–8.
Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16.
Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61.
Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59:709–17.
Brusselle G, Michils A, Louis R, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009;103:1633–42.
Grimaldi-Bensouda L, Zureik M, Aubier M, et al. Pharmacoepidemiology of asthma and xolair (PAX) study group. Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest. 2013;143:398–405.
Korn S, Thielen A, Seyfried S, Taube C, Kornmann O, Buhl R. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med. 2009;103:1725–31.
Molimard M, de Blay F, Didier A, Le Gros V. Effectiveness of omalizumab (Xolair) in the first patients treated in real-life practice in France. Respir Med. 2008;102:71–6.
Tzortzaki EG, Georgiou A, Kampas D, et al. Long-term omalizumab treatment in severe allergic asthma: the South-Eastern Mediterranean “real-life” experience. Pulm Pharmacol Ther. 2012;25:77–82.
Lafeuille MH, Dean J, Zhang J, Duh MS, Gorsh B, Lefebvre P. Impact of omalizumab on emergency-department visits, hospitalizations, and corticosteroid use among patients with uncontrolled asthma. Ann Allergy Asthma Immunol. 2012;109:59–64.
Bousquet J, Siergiejko Z, Swiebocka E, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66:671–8.
Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–15.
Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107:1141–51.
Braunstahl GJ, Leo J, Thirlwell J, Peachey G, Maykut R. Uncontrolled persistent allergic asthma in practice: eXpeRience registry baseline characteristics. Curr Med Res Opin. 2011;27:761–7.
Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol. 2001;107:3–8.
Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J. 2002;19:61–7.
European Respiratory Society, European Lung Foundation (2003) European lung white book; The first comprehensive survey on respiratory health in Europe, Sheffield.
Accordini S, Corsico AG, Braggion M, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013;160:93–101.
Accordini S, Corsico A, Cerveri I, et al. Therapy and health economics working group of the European community respiratory health survey II. The socio-economic burden of asthma is substantial in Europe. Allergy. 2008;63:116–24.
Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–35.
Costello RW, Long DA, Gaine S, Mc Donnell T, Gilmartin JJ, Lane SJ. Therapy with omalizumab for patients with severe allergic asthma improves asthma control and reduces overall healthcare costs. Ir J Med Sci. 2011;180:637–41.
Guy-Alfandary S, Nahir B, Namer-Tal Y, Raz M. Clinical utilization pattern and effectiveness of omalizumab for asthma patients in Israel. Chest. 2012;142(4_MeetingAbstracts):707A.
Cazzola M, Camiciottoli G, Bonavia M, et al. Italian real-life experience of omalizumab. Respir Med. 2010;104:1410–6.
Korn S, Schumann C, Kropf C, et al. Effectiveness of omalizumab in patients 50 years and older with severe persistent allergic asthma. Ann Allergy Asthma Immunol. 2010;105:313–9.
Milgrom H, Berger W, Nayak A, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001;108:E36.
Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open-label study. Respir Med. 2008;102:1371–8.
Sykes A, Johnston SL. Etiology of asthma exacerbations. J Allergy Clin Immunol. 2008;122:685–8.
US Department of Health and Human Services, National Institute of Health. NIH news: world asthma day, May 5, 2009. http://www.nih.gov/news/health/may2009/niaid-05.htm. Accessed 20 Oct 2014.
Montana Department of Public Health and Human Services Public Health and Safety Division. State Public Health Assessment, Montana, 2012. http://www.astho.org/accreditation/montana-state-health-assessment/. Accessed 22 Oct 2014.
Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100:1139–51.
Acknowledgments
Sponsorship for the registry and article processing charges were funded by Novartis Pharma AG, Basel, Switzerland. All authors had full access to all of the data in this registry and take complete responsibility for the integrity of the data and accuracy of the data analysis. Medical writing assistance and assistance in the preparation of the manuscript was provided by Santosh Tiwari (Novartis Pharma AG, Basel, Switzerland) and Richard Crampton (Novartis Pharma AG, Basel, Switzerland), and was funded by Novartis Pharma AG, Basel, Switzerland. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Gert-Jan Braunstahl has received research grant/support for consultations and/or speaking at conferences from Novartis Pharma AG, Basel Switzerland; GlaxoSmithKline, London, UK; AstraZeneca, London, UK; and MSD, Lucerne, Switzerland. At the time of the registry Janice Canvin, Guy Peachey and Panayiotis Georgiou were employees of Novartis Pharmaceuticals UK Limited, Horsham, West Sussex, UK. Chien-Wei Chen is an employee of Novartis Pharmaceuticals Corporation, NJ, USA.
Compliance with ethical guidelines
In accordance with local regulations, the registry design and amendments were reviewed by independent ethics committees or institutional review boards at each participating centre. All procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients included in the registry.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Braunstahl, GJ., Canvin, J., Peachey, G. et al. Healthcare Resource Utilization in Patients Receiving Omalizumab for Allergic Asthma in a Real-World Setting. Biol Ther 4, 57–67 (2014). https://doi.org/10.1007/s13554-014-0019-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13554-014-0019-z