Abstract
Background
In chronic heart failure (CHF), cachexia is a hallmark of the terminal stage of this disease and is associated with a severely reduced quality of life and poor prognosis. Therapeutic options are currently not available. Ghrelin and its analogues BIM-28125 and BIM-28131 (now known as RM-131) have been shown to increase weight in a rat model of CHF. It has been further demonstrated that the expression of myostatin, a negative regulator of skeletal muscle mass, is increased in CHF. The aim of the study was to investigate the influence of ghrelin or its analogues on myostatin in CHF.
Methods
In an animal model of CHF, Sprague–Dawley rats received either ghrelin or two ghrelin analogues BIM-28125 and BIM-28131 in two different concentrations (50 and 500 nmol/kg/day) compared to placebo. The compounds were delivered using osmotic mini pumps. The expression of myostatin was analyzed in skeletal muscle by RT-PCR and Western blot, and muscle mass of gastrocnemius muscle was measured. The plasma levels of tumor necrosis factor alpha (TNF-α) were measured.
Results
The relative weight of the gastrocnemius muscle of the sham-operated group was significantly increased compared to placebo-treated CHF rats. The application of ghrelin analogue BIM-28125 and BIM-28131 in their higher concentrations led to a significant reduction in myostatin mRNA expression in comparison to placebo. Myostatin protein expression was significantly reduced in both concentrations of ghrelin and BIM-28131 and in the lower concentration of BIM-28125. The increase of TNF-α plasma concentration in the CHF-animals could be abolished by all used substances.
Conclusions
In an animal model of CHF, the expression of myostatin is significantly reduced in the skeletal muscle after application of ghrelin and most concentrations of its analogues BIM-28125 and BIM-28131 possibly due to anti-inflammatory effects.
Similar content being viewed by others
1 Introduction
In western countries, chronic heart failure (CHF) is one of the most common causes of death, and more than 50 % of patients diagnosed with CHF will die within 5 years. Cardiac cachexia, which occurs in approximately 15 % of these patients, shortens this period to 18 month [1]. Cachexia is a wasting disorder which affects not only fat but also lean muscle mass. By definition, a nonoedematous weight loss of more than 5 % over a period of 3–12 months characterizes this catabolic state [2]. So far, therapeutic options are eagerly expected, but no alleviating agent has been found yet.
Myostatin, a key regulator of skeletal muscle mass and a member of the transforming growth factor-β family is increased in a number of cachectic states [3–5]. Cell culture, animal, and human studies have demonstrated increased concentrations in CHF possibly driven by proinflammatory cytokines like TNF-α which return to baseline levels by endurance exercise training [6, 7]. Furthermore, systemic administration of this negative growth regulator leads to muscle wasting in mice [8]. It is found predominantly not only in skeletal but also in heart muscle [9, 10].
Ghrelin is a 28-amino acid hormone whose discovery was based on the ability for it to bind to the growth hormone secretagogue receptor 1a (GHSR-1a) in the hypothalamus and stimulate growth hormone release [11]. Growth hormone (GH) and insulin-like growth factor 1 (IGF-1) are important physiological regulators of myocardial growth and performance [12]. Patients with CHF show typically elevated serum levels of GH and normal to decreased circulating levels of IGF-1 [13, 14], but decreased IGF-1 levels in the skeletal muscle. This indicates a pathological GH/IGF-1 axis which potentially contributes to the syndrome of cachexia. Treatment of catabolic states and cachexia with ghrelin has demonstrated promising results [15–18]. Human clinical trials conducted with native ghrelin in patients with cardiac cachexia demonstrate increases in appetite, weight, and cardiac output without apparent toxicity [15]. In a previous study, it could be shown that ghrelin and the related analogues BIM-28125 and BIM-28131 (now known as RM-131) induced weight gain in an animal model of CHF. BIM-28131 seemed to be superior in inducing a balanced weight gain of fat and lean tissue while normalizing the expression of muscle ring finger 1 and muscle atrophy F-box [19]. To our knowledge, it is yet undiscovered if ghrelin has any effects on myostatin. The present study was therefore conducted to assess the regulation of myostatin in this animal model of ligation of the left anterior descending coronary artery (LAD) after the application of ghrelin and its analogues BIM-28125 and BIM-28131.
2 Methods
2.1 Rat model of CHF, necropsy, and measurement of gastrocnemius muscle
Male Sprague–Dawley rats underwent LAD ligation to induce myocardial infarction or were sham operated (Harlan Winkelmann, Borchen, Germany). Sham operation consisted of a thoracotomy and cardiac exposure without LAD ligation. Surgery was performed as described in detail by Palus et al. [19]. A scheme of the study design is outlined in Fig. 1. The mortality rate was 31 % after 24 h. Two weeks after the operation, diuretics were added to the drinking water (furosemide 86 mg/l). Four weeks after the operation, the animals were randomized in seven treatment groups (n = 18 each). Rats were given either placebo (CHF placebo), human ghrelin, or one of the ghrelin compounds BIM-28125 and BIM-28131 at 50 or 500 nmol/kg/day (IPSEN Pharmaceuticals, Milford, MA, USA) via osmotic mini pumps (Charles River, Sulzfeld, Germany). Sham animals received placebo (n = 14). The pumps which were implanted subcutaneously on the back had to be replaced on the 42nd day. Animals were sacrificed on the 56th day and had to be excluded when the infarct size of the left ventricle was less than 25 % (n = 5). The harvested gastrocnemius muscle was weighted and immediately snap frozen in liquid nitrogen and stored at −80 °C. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85–23, revised 1996). The local ethics committee (Landesamt für Gesundheit und Soziales Berlin, Germany, permit number G 0116/05) accepted the experimental protocols.
2.2 Quantification of muscle weight
The weight of the gastrocnemius muscle was calculated as a relative weight to the length of the tibia to avoid interindividual confounders. Tibia length was measured via ultrasound.
2.3 Quantification of myostatin mRNA
Total RNA was isolated from gastrocnemius muscle tissue using RNeasy (Qiagen, Hilden, Germany). An aliquot of total RNA was reverse-transcribed into cDNA using random hexamers and Sensiscript reverse transcriptase (Qiagen, Hilden, Germany). For quantitative RT-PCR, 1 μl of the cDNA was used applying the LightCycler system (Roche Diagnostics Inc.). For the detection of myostatin, specific primers and internal probes were used. The expression of specific genes was normalized to the expression of 18S rRNA. The following primers and conditions were used:
18S rRNA (5′-ATACAGGACTCTTTCGAGGCCC-3′, 5′-CGGGACACTCAGCTAAGAGCAT-3′; 61 °C annealing), myostatin (5′-GTCTTCACATCAATACTCTGCCA-3′, 5′-CATGCCTACCGAGTCTGACTT-3′; 55 °C annealing), and myostatin probes (5′-LC640-GTGCAAATCCTGAGACTCATCAAACCCATG–PH-3′, 5’-GAGAGCCGTCAAGACT CCTACAACAGTGT–FL-3′).
2.4 Quantification of myostatin protein expression
Frozen tissue samples were homogenized in lysis buffer [20] and Western blot analysis was performed as described previously [21]. Myostatin protein expression was quantified using specific antibodies (R&D Systems, Heidelberg, Germany). To compensate for blot-to-blot variations, an internal standard was loaded on each SDS–polyacrylamide gel, and the densitometry results were expressed as the ratio between the sample and the standard intensity. Loading differences were controlled by reprobing the blot with an antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH; HyTest, Turku, Finland). All samples were analyzed in duplicate.
2.5 Quantification of plasma TNF-α
Plasma levels of TNF-α were measured by high-sensitivity ELISA assay (Hölzel Diagnostika, Köln, Germany). Results were compared with standard curves, and the lower detection limit for TNF-α was <10 pg/ml with an inter- and intra-assay variability <8 %.
2.6 Statistical analysis
Values are given as mean ± SEM for all variables. Intergroup comparisons were performed with a one-way ANOVA followed by a Tukey post hoc test using GraphPad InStat version 3.01 software. A probability value of <0.05 was considered statistically significant.
3 Results
3.1 Gastrocnemius muscle weight
Measurement of the gastrocnemius muscle revealed no differences between the placebo-treated rats and animals which received ghrelin and ghrelin analogues although there was a trend toward higher muscle mass especially in the high-dose compounds (Fig. 2). As expected, the weight of the gastrocnemius muscle of the sham-operated group was significantly increased compared to CHF placebo-treated rats (504.4 ± 32.9 mg vs. 470.9 ± 38.7 mg, p < 0.05; Fig. 2).
3.2 Expression of myostatin mRNA in the gastrocnemius muscle after induction of heart failure
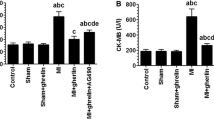
In rats treated with higher doses of the ghrelin analogues BIM-28125 and BIM-28131, the mRNA transcription of myostatin was significantly reduced compared to CHF placebo-treated animals (0.033 ± 0.007 and 0.038 ± 0.008, respectively, vs. 0.106 ± 0.015 arbitrary units, p < 0.05; Fig. 3). As expected sham-operated animals had significantly lower levels of mRNA compared to the CHF placebo group (0.041 ± 0.008 vs. 0.106 ± 0.015 arbitrary units, p < 0.05).
Regulation of myostatin mRNA in gastrocnemius muscle after induction of CHF by myocardial infarction. Both analogues BIM-28125 and BIM-28131 were able to reduce mRNA expression significantly in the high-dose application (500 nmol/kg/day) in comparison to placebo-treated CHF rats. The values are depicted as ratio over the expression of 18S rRNA. Results are depicted as means ± SEM. *p ≤ 0.05 vs. placebo-treated CHF rats
3.3 Expression of myostatin protein in the gastrocnemius muscle after induction of heart failure
Myostatin protein expression was with one exception (BIM-28125 500 nmol/kg/day) significantly downregulated in animals receiving ghrelin or ghrelin analogues in different concentrations in comparison to placebo-treated animals (BIM-28125 50 nmol/kg/day 0.72 ± 0.06, p < 0.001; BIM-281231 50 nmol/kg/day 1.22 ± 0.12, p < 0.01; BIM-28131 500 nmol/kg/day 1.26 ± 0.13, p < 0.05; ghrelin 50 nmol/kg/day 1.15 ± 0.12, p < 0.01; ghrelin 500 nmol/kg/day 1.24 ± 0.17, p < 0.05; all vs. CHF placebo 2.03 ± 0.2 arbitrary units; Fig. 4).
Regulation of myostatin protein in gastrocnemius muscle after induction of CHF by myocardial infarction. Apart from BIM-28125 500 μg, all other components including ghrelin decreased myostatin protein concentration in skeletal muscle significantly in comparison to placebo-treated CHF rats. The values are depicted as ratio over the expression of GAPDH. Results are depicted as means ± SEM. ***p < 0.001, **p < 0.01, and *p < 0.05 vs. placebo-treated CHF rats
As seen on mRNA level, sham-operated animals had significantly lower myostatin concentrations compared to the CHF placebo group (1.25 ± 0.19 vs. 2.03 ± 0.2 arbitrary units, p < 0.05).
3.4 Plasma TNF-α concentration after induction of heart failure
TNF-α concentration in the plasma increased significantly in the CHF placebo group compared to the sham placebo animals (15.81 ± 0.93 vs. 5.69 ± 0.28 arbitrary units, p < 0.001). This impact of CHF induction on TNF-α concentration was completely abolished in all groups treated with either ghrelin or its analogues (Fig. 5).
4 Discussion
Cardiac cachexia is a devastating complication of CHF associated with high morbidity and mortality rates. Myostatin, a negative regulator of muscular growth, is upregulated in cachexia [3–7]. Ghrelin, a potent stimulator of growth hormone, has demonstrated positive effects in cachectic states [15–18]. However, the influence of ghrelin on myostatin is unknown at this moment and was therefore investigated in this study. Three important messages emerge from this study: First, as a proof of concept, it could be shown that 8 weeks after myocardial infarction, muscle wasting is detectable in this animal model. Second, ghrelin and the ghrelin analogues BIM-28125 and BIM-28131 significantly reduced the expression of myostatin on protein levels. Third, in CHF animals, TNF-α is downregulated to baseline levels due to treatment with all investigated substances.
4.1 The role of myostatin in skeletal muscle in CHF and cachexia
The first report on myostatin in CHF published by Shyu and colleagues showed an upregulation of myocardial myostatin mRNA and protein in a rat model of volume-overload heart failure induced by an aortocaval shunt. Assessment of the cross-sectional area of the semimembranous muscle in the rat hind limb revealed skeletal muscle wasting in five of eight animals with volume overload CHF [22]. This finding was supported by an animal study of ischemic cardiomyopathy where a significant increase in myostatin protein expression in heart and skeletal muscle was observed after LAD ligation. Interestingly, these effects could be reversed by 4 weeks of exercise training on a treadmill [6]. Recently, it could be demonstrated in a clinical trial that late-stage heart failure patients express significantly higher levels of myostatin which were also reversible by exercise endurance training over 4 weeks [7].
4.2 The role of ghrelin in CHF and cachexia
While endogenous ghrelin levels are increased in the setting of cachexia [23], treatment with ghrelin and other GHSR-1a agonists in animal models of cachexia and in humans with cachexia has demonstrated consistent effects of increased appetite and improved weight gain [24]. The GH-releasing effect of ghrelin has been shown to be more potent than that of GH-releasing hormone [25]. As in other terminal diseases underlying cachexia, administration of ghrelin and ghrelin agonists in animal models of CHF has been shown to improve accrual of lean mass, fat mass, and body weight several fold vs. placebo over 3–4 weeks of treatment periods [26, 27]. In a report by Nagaya and coworkers, it has been proven that only a 3-week administration led to a significant increase in left ventricular ejection fraction, exercise capacity, and muscle wasting in patients with CHF [15]. Growth hormone and IGF-dependent mechanisms resulting in a decrease in LV-wall stress as well as an increase in lean mass and muscle strength were believed to be responsible for this improvement.
4.3 The possible relationship between myostatin, ghrelin, GH, and IGF
In the setting of CHF, there are increased circulating ghrelin levels on one side, reduced levels of IGF-1 [28], and elevated myostatin levels in the skeletal muscle on the other side [7]. This catabolic situation within the skeletal muscle, which progresses untreated to cachexia so far, can only be reversed by a few interventions like exercise training [7, 29]. It has been shown that chronic ghrelin administration increases circulating levels of IGF-1 [27, 30]. But the stimulatory effect of ghrelin or ghrelin receptor agonists on the GH–IGF-1 system does not last very long. It has been postulated that ghrelin is not physiologically involved in the regulation of GH, since circulating levels of ghrelin are not correlated with those of GH either in physiological or in pathological conditions [31]. The explanation of the discrepancy between the GH–IGF-1 system and ghrelin are the so-called GH-independent and pleiotropic effects like protection of the heart from ischemia–reperfusion damage [32, 33].
Another pleiotropic effect of ghrelin and ghrelin receptor agonists is its influence on anti-inflammatory cytokines [34]. This anti-inflammatory effect does not depend on pituitary GH secretion which has been proven in cell culture experiments with endothelial or immune cells [35, 36] where ghrelin or ghrelin receptor agonists were able to decrease cytokine production. GH in contrast has a stimulatory effect on cytokine activity [37].
In the present study, we could demonstrate that myostatin decreases after therapy with ghrelin or ghrelin analogues. On mRNA level, only the highest concentrations of the two ghrelin analogues led to significantly reduced myostatin which underlines the dose-dependent effect and supports the hypothesis that posttranscriptional and posttranslational modifications are responsible for the significant changes on protein level. The reason why BIM-28125 500 nmol/kg/day did not decrease the myostatin protein expression levels at the large dose is not entirely understood. Although there was a potent effect on myostatin mRNA, myostatin protein was not diminished. One possible explanation could be an impact of BIM-28125 on myostatin inhibitors in higher concentrations which might be downregulated leading to higher myostatin protein concentrations. Alternatively, BIM-28125 could have direct effects on the myostatin translation rate in higher concentrations.
The impact of ghrelin and its analogues BIM-28125 and BIM-28131 on myostatin seems to be another pleiotropic effect of ghrelin. One possible explanation for the myostatin downregulation could be the above-mentioned reduction of TNF-α level. This is supported by a previous study, which demonstrated significantly increased levels of myostatin mRNA and protein levels after incubation of C2C12 myocytes with TNF-α via NF-κB and p38 MAPK activation [6]. In the gastrocnemius muscle of CHF animals, TNF-α mRNA levels were significantly higher compared with controls, which is in agreement with previously published data [38]. Furthermore, there was a significant correlation between these raised TNF-α levels and myostatin concentration, supporting the hypothesis of a relationship between TNF-α and myostatin. To evaluate the anti-inflammatory effect of ghrelin and its analogues in the present study, plasma TNF-α levels were measured. Interestingly, the significant rise of TNF-α in CHF rats was blunted completely by all investigated substances underlining the anti-inflammatory capacity of ghrelin as well as BIM-28125 and BIM-28131. Although the direct pathway of myostatin stimulation by ghrelin and its analogues BIM-28125 and BIM-28131 is still undiscovered, the anti-inflammatory potential could be one plausible explanation.
In summary, the induction of CHF led to a significant loss of relative weight of the gastrocnemius muscle and to an increase in myostatin protein expression. Administration of ghrelin and its analogues BIM-28125 and BIM-28131 significantly reduced these levels back to baseline values. This beneficial anticatabolic effect could involve the anti-inflammatory potential of ghrelin which needs further investigation.
References
Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3.
Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9.
Costelli P, Muscaritoli M, Bonetto A, Penna F, Reffo P, Bossola M, et al. Muscle myostatin signalling is enhanced in experimental cancer cachexia. Eur J Clin Invest. 2008;38:531–8.
Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, et al. Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA. 1998;95:14938–43.
Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging. 2002;6:343–8.
Lenk K, Schur R, Linke A, Erbs S, Matsumoto Y, Adams V, et al. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail. 2009;11:342–8.
Lenk K, Erbs S, Höllriegel R, Beck E, Linke A, Gielen S, et al. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2012;19(3):404–11.
Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–8.
McPherron AC, Lawler AM, Lee S-J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90.
Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, et al. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180(1):1–9.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60.
Duerr RL, McKirnan MD, Gim RD, Clark RG, Chien KR, Ross Jr J. Cardiovascular effects of insulin-like growth factor-1 and growth hormone in chronic left ventricular failure in the rat. Circulation. 1996;93(12):2188–96.
Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–34.
Niebauer J, Pflaum CD, Clark AL, Strasburger CJ, Hooper J, Poole-Wilson PA, et al. Deficient insulin-like growth factor I in chronic heart failure predicts altered body composition, anabolic deficiency, cytokine and neurohormonal activation. J Am Coll Cardiol. 1998;32:393–7.
Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110(24):3674–9.
Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128:1187–93.
Ashby DR, Ford HE, Wynne KJ, Wren AM, Murphy KG, Busbridge M, et al. Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int. 2009;76:199–206.
Strasser F, Lutz TA, Maeder MT, Thuerlimann B, Bueche D, Tschop M, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008;98:300–8.
Palus S, Schur R, Akashi YJ, Bockmeyer B, Datta R, Halem H, et al. Ghrelin and its analogues, BIM-28131 and BIM-28125, improve body weight and regulate the expression of MuRF-1 and MAFbx in a rat heart failure model. PLoS ONE. 2011;6(11):e26865.
Adams V, Linke A, Kränkel N, Erbs S, Gielen S, Möbius-Winkler S, et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Ciculation. 2005;111:555–62.
Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, et al. Antioxidative effects of exercise training in patients with chronic heart failure. Increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–70.
Shyu KG, Lu MJ, Wang BW, Sun HY, Chang H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur J Clin Invest. 2006;36(10):713–9.
Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–8.
DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148(6):3004–12.
Takaya K, Ariyasu H, Kanamoto N, et al. Ghrelin strongly stimulates growth hormone (GH) release in humans. J Clin Endocrinol Metab. 2000;85:4908–11.
Akashi YJ, Palus S, Datta R, Halem H, Taylor JE, Thoene-Reineke C, et al. No effects of human ghrelin on cardiac function despite profound effects on body composition in a rat model of heart failure. Int J Cardiol. 2009;137:267–75.
Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–5.
Schulze PC, Gielen S, Adams V, Linke A, Mobius-Winkler S, et al. Muscular levels of proinflammatory cytokines correlate with a reduced expression of insulinlike growth factor-I in chronic heart failure. Basic Res Cardiol. 2003;98:267–74.
Hambrecht R, Schulze PC, Gielen S, Linke A, Möbius-Winkler S, Erbs S, et al. Effects of exercise training on insulin-like growth factor-I expression in the skeletal muscle of non-cachectic patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2005;12(4):401–6.
Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13.
Torsello A, Bresciani E, Avallone R, Locatelli V. Ghrelin and GH secretion. Minerva Endocrinol. 2002;27:257–64.
De Gennaro Colonna V, Rossoni G, Bernareggi M, Muller EE, Berti F. Cardiac ischemia and impairment of vascular endothelium function in hearts from growth hormone-deficient rats: protection by hexarelin. Eur J Pharmacol. 1997;334:201–7.
Locatelli V, Rossoni G, Schweiger F, Torsello A, De Gennaro Colonna V, Bernareggi M, et al. Growth hormone-independent cardioprotective effects of hexarelin in the rat. Endocrinology. 1999;140:4024–31.
Granado M, Priego T, Martín AI, Villanúa MA, López-Calderón A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288:E486–92.
Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66.
Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–6.
Uronen-Hansson H, Allen ML, Lichtarowicz-Krynska E, Aynsley-Green A, Cole TJ, Hoiden-Guthenberg I, et al. Growth hormone enhances proinflammatory cytokine production by monocytes in whole blood. Growth Horm IGF Res. 2003;13:282–6.
Gielen S, Adams V, Möbius-Winkler S, Linke A, Erbs S, Yu J, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42(5):861–8.
von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.
Acknowledgments
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [39].
Conflict of interest
The authors have the following competing interest: Rakesh Datta, Jesse Dong, and Michael D. Culler are employees of IPSEN Pharmaceuticals.
Funding
This study was funded by IPSEN Pharmaceuticals through the employment of authors Rakesh Datta, Jesse Dong, and Michael D. Culler, who contributed to all aspects of the study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lenk, K., Palus, S., Schur, R. et al. Effect of ghrelin and its analogues, BIM-28131 and BIM-28125, on the expression of myostatin in a rat heart failure model. J Cachexia Sarcopenia Muscle 4, 63–69 (2013). https://doi.org/10.1007/s13539-012-0085-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13539-012-0085-3