Abstract
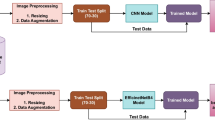
Bones during growth period undergo substantial changes in shape and size. X-ray imaging has been routinely used for bone growth diagnosis purpose. Hand has been the part of choice for X-ray imaging due to its high bone parts count and relatively low radiation requirement. Traditionally, bone age estimation has been performed by referencing atlases of images of hand bone regions where aging-related metamorphoses are most conspicuous. Tanner and Whitehouse’ and Greulich and Pyle’s are some well known ones. The process entails manual comparison of subject’s hand region images against a set of corresponding images in the atlases. It is desired to estimate bone age from hand images in an automated manner, which would facilitate more efficient estimation in terms of time and labor cost and enables quantitative and objective assessments. Deep learning method has proved to be a viable approach in a number of application domains. It is also gaining wider grounds in medical image analysis. A cascaded structure of layers can be trained to mimic the image-based cognitive and inference processes of human and other higher organisms. We employed a set of well known deep learning network architectures. In the current study, 3000 images were manually curated to mark feature points on hands. They were used as reference points in removing unnecessary image regions and to retain regions of interest (ROI) relevant to age estimation. Different ROI’s were defined and used—that of rather small area mostly made up of carpal and metacarpal bones and that includes most of phalanges in addition. Irrelevant intensity variation across cropped images was minimized by applying histogram equalization. In consideration of the established gender difference in growth rates, separate gender models were built. Certain age range image data are far scarcer and exhibit rather large excursion in morphology from other age ranges—e.g. infancy and very early childhood. Many studies excluded them and addressed only elder subjects in later developmental stages. Considering infant age group’s diagnosis demand is just as valid as elder groups’, we included entire age ranges for our study. A number of different deep learning architectures were trained with varying region of interest definitions. Smallest mean absolute difference error was 8.890 months for a test set of 400 images. This study was preliminary, and in the future, we plan to investigate alternative approaches not taken in the present study.









Similar content being viewed by others
References
Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, CA: Stanford University Press; 1959.
Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51(3):170–9.
Visual dictionary [cited at 2018 Jan 5]. http://visual.merriam-webster.com/human-being/anatomy/skeleton/hand.php.
RSNA. Pediatric Bone Age Challenge. IL, USA: RSNA [cited at 2017 Dec 13]. http://rsnachallenges.cloudapp.net/competitions/4.
Vicente G, Ratib O. Hand bone age: a digital atlas of skeletal maturity. Berlin: Springer; 2005.
Gaskin CM, Kahn SL, Bertozzi JC, Bunch PM. Skeletal development of the hand and wrist: a radiographic atlas and digital bone age companion. Oxford: Oxford University Press; 2011.
Thodberg HH, Kreiborg S, Juul A, Pedersen KD. The BoneXpert method for automated determination of skeletal maturity. IEEE Trans Med Imaging. 2009;28(1):52–66 (abstract).
Kim JR. Computerized bone age estimation using deep learning based program: evaluation of the accuracy and efficiency. Am J Roentgenol. 2017;209(6):1374–80. https://doi.org/10.2214/AJR.17.18224
Spampinato C, Palazzo S, Giordano D, Aldinucci M, Leonardi R. Deep learning for automated skeletal bone age assessment in X-ray images. Med Image Anal. 2016;36:41–51.
Wikipedia. Backpropagation. Wikipedia; c2017 [cited at 2017 Dec 11]. https://en.wikipedia.org/wiki/Backpropagation.
Srivastava Nitish, Hinton Geoffrey, Krizhevsky Alex, Sutskever Ilya, Salakhutdinov Ruslan. Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res. 2014;15:1929–58.
BAIR. Caffe Installation. CA, USA: BAIR [cited at 2017 Dec 13]. http://caffe.berkeleyvision.org/installation.html.
BVLC/caffe. c2017 [cited 2017 Dec 13]. github.com/BVLC/caffe/tree/master/models/bvlc_reference_caffenet.
Szegedy C, Liu W, Jia Y, Sermanet P, Reed S, Anguelov D, Erhan D, Vanhoucke V, Rabinovich A. https://arxiv.org/abs/1409.4842.
He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. https://arxiv.org/abs/1512.03385.
Lawrence I-Kuei Lin. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989;45(1):255–268. https://doi.org/10.2307/2532051.JSTOR2532051.
Deng J, Dong W, Socher R, Li LJ, Li K et al. ImageNet: a large-scale hierarchical image database. In: Proceedings of IEEE conference on computer vision and pattern recognition. 2009.
Ontell FK, Ivanovic M, Ablin DS, Barlow TW. Bone age in children of diverse ethnicity. AJR. 1996;167:1395–8.
Zhang A, Sayre JW, Vachon L, Liu BJ, Huang HK. Racial differences in growth patterns of children assessed on the basis of bone age. Radiology. 2009;250:228–35.
Romany FM. Deep-learning-based automatic computer-aided diagnosis system for diabetic retinopathy. Biomed Eng Lett. 2017;8(1):41–57.
Salamon J, Bello JP. Deep convolutional neural networks and data augmentation for environmental sound classification, 2016, IEEE Signal Proc Lett.
Acknowledgements
This work was partly supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government(MSIT) (No. 2019-0-01750, Development of an optimal limb-compressing cardiovascular tratment using deep learning technique) and Gachon University Gil Medical Center (2018-5283), the GRRC program of Gyeonggi province (GRRC Gachon 2017-B01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical statement
The research is done following all the ethics guidelines provided by the Springer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J.H., Kim, Y.J. & Kim, K.G. Bone age estimation using deep learning and hand X-ray images. Biomed. Eng. Lett. 10, 323–331 (2020). https://doi.org/10.1007/s13534-020-00151-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-020-00151-y