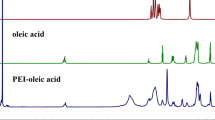
Abstract
Gold nanoparticles (AuNPs) play an important role in catalytic reduction of 4-nitrophenol. To obtain highly dispersed and stable AuNPs, the stabilizer is required. In this work, core-shell-corona polymeric micelles prepared by self-assembly of the commercial ABC triblock polymer poly(ethylene oxide-b-2-vinyl pyridine-b-styrene) (abbreviated to PEO-b-P2VP-b-PS) were explored as an excellent stabilizer of AuNPs. PEO-b-P2VP-b-PS-stabilized AuNPs with the diameter of approximately 7 nm were prepared through chemical reduction of Au ions in aqueous medium at mild conditions. The preparation was environmentally friendly without using any organic solvents and suitable for commercial process without heat. PEO-b-P2VP-b-PS-stabilized AuNPs had high catalytic activity for chemical reduction of 4-nitrophenol. The results of stability and catalytic activity indicated that nitrogen atoms and triblock structure of PEO-b-P2VP-b-PS had synergistic effect on stabilizing AuNPs.






Similar content being viewed by others
References
Zhang C, Jiang YH, Li YL, Hu ZX, Zhou L, Zhou MH (2013a) Three-dimensional electrochemical process for wastewater treatment: a general review. Chem Eng J 228:455–467. doi:10.1016/j.cej.2013.05.033
Guo PC, Tang L, Tang J, Zeng GM, Huang BB, Dong HR, Zhang Y, Zhou YY, Deng YC, Ma LL, Tan SR (2016) Catalytic reduction-adsorption for removal of p-nitrophenol and its conversion p-aminophenol from water by gold nanoparticles supported on oxidized mesoporous carbon. J Colloid Interface Sci 469:78–85. doi:10.1016/j.jcis.2016.01.063
Zhao PX, Feng XW, Huang DS, Yang GY, Astruc D (2015) Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord Chem Rev 287:114–136. doi:10.1016/j.ccr.2015.01.002
Lin C, Tao K, Hua DY, Ma Z, Zhou SH (2013) Size effect of gold nanoparticles in catalytic reduction of p-nitrophenol with NaBH4. Molecules 18:12609–12620. doi:10.3390/molecules181012609
Hansen TW, Delariva AT, Challa SR, Datye AK (2013) Sintering of catalytic nanoparticles: particle migration or ostwald ripening? Acc Chem Res 46:1720–1730. doi:10.1021/ar3002427
Pachon LD, Rothenberg G (2008) Transition-metal nanoparticles: synthesis, stability and the leaching issue. Appl Organomet Chem 22:288–299. doi:10.1002/aoc.1382
Dimitratos N, Villa A, Prati L, Hammond C, Chan-Thaw CE, Cookson J, Bishop PT (2016) Effect of the preparation method of supported Au nanoparticles in the liquid phase oxidation of glycerol. Appl Catal A-Gen 514:267–275. doi:10.1016/j.apcata.2015.12.031
Kyriakidou EA, Khivantsev K, Gostanian TM, Alexeev OS, Amiridis MD (2015) Silica-supported gold/dendrimer nanocomposites with controlled sizes of gold particles. Appl Catal A-Gen 504:482–492. doi:10.1016/j.apcata.2014.11.022
Deng HJ, Wang SX, Jin S, Yang S, Xu YJ, Liu LL, Xiang J, Hu DQ, Zhu MZ (2015) Active metal (cadmium) doping enhanced the stability of inert metal (gold) nanocluster under O-2 atmosphere and the catalysis activity of benzyl alcohol oxidation. Gold Bull 48:161–167. doi:10.1007/s13404-015-0174-0
Xia YY, Wan JM, Gu QF (2011) Silk fibroin fibers supported with high density of gold nanoparticles: fabrication and application as catalyst. Gold Bull 44:171–176. doi:10.1007/s13404-011-0024-7
Wan DC, Fu Q, Huang JL (2006) Synthesis of amphiphilic hyperbranched polyglycerol polymers and their application as template for size control of gold nanoparticles. J Appl Polym Sci 101:509–514. doi:10.1002/app.23309
Zhang H, Cui H (2009) Synthesis and characterization of functionalized ionic liquid-stabilized metal (gold and platinum) nanoparticles and metal nanoparticle/carbon nanotube hybrids. Langmuir 25:2604–2612. doi:10.1021/la803347h
Wang JC, Neogi P, Forciniti D (2006) On one-dimensional self-assembly of surfactant-coated nanoparticles. J Chem Phys 125:194717. doi:10.1063/1.2375091
Pettibone JM, Hudgens JW (2011) Gold cluster formation with phosphine ligands: etching as a size-selective synthetic pathway for small clusters? ACS Nano 5:2989–3002. doi:10.1021/nn200053b
Mandal D, Ghosh M, Maiti S, Das K, Das PK (2014) Water-in-oil microemulsion doped with gold nanoparticle decorated single walled carbon nanotube: scaffold for enhancing lipase activity. Colloid Surf B-Biointerfaces 113:442–449. doi:10.1016/j.colsurfb.2013.09.047
Zhang JG, Yuan Y, Kilpin KJ, Kou Y, Dyson PJ, Yan N (2013b) Thermally responsive gold nanocatalysts based on a modified poly-vinylpyrrolidone. J Mol Catal A-Chem 371:29–35. doi:10.1016/j.molcata.2013.01.030
Crooks RM, Zhao MQ, Sun L, Chechik V, Yeung LK (2001) Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc Chem Res 34:181–190. doi:10.1021/ar000110a
Chen J, Xiao P, Gu JC, Han D, Zhang JW, Sun AH, Wang WQ, Chen T (2014) A smart hybrid system of Au nanoparticle immobilized PDMAEMA brushes for thermally adjustable catalysis. Chem Commun 50:1212–1214. doi:10.1039/c3cc47386d
Huang X, Li BY, Zhang H, Hussain I, Liang LY, Tan BE (2011) Facile preparation of size-controlled gold nanoparticles using versatile and end-functionalized thioether polymer ligands. Nano 3:1600–1607. doi:10.1039/c0nr00835d
Dai Y, Li Y, Wang SP (2015) ABC triblock copolymer-stabilized gold nanoparticles for catalytic reduction of 4-nitrophenol. J Cata 329:425–430. doi:10.1016/j.jcat.2015.06.006
Dai Y, Zhang X, Zhuo R (2016a) Amphiphilic linear-hyperbranched polymer poly(ethylene glycol)-branched polyethylenimine-poly(ϵ-caprolactone) synthesis, self-assembly and application as stabilizer of platinum nanoparticles. Polym Int 65:691–697. doi:10.1002/pi.5118
Dai Y, Yu P, Zhang XJ, Zhuo RX (2016b) Gold nanoparticles stabilized by amphiphilic hyperbranched polymers for catalytic reduction of 4-nitrophenol. J Cata 337:65–71. doi:10.1016/j.jcat.2016.01.014
Li YQ, Bastakoti BP, Malgras V, Li CL, Tang J, Kim JH, Yamauchi Y (2015) Polymeric micelle assembly for the smart synthesis of mesoporous platinum nanospheres with tunable pore sizes. Angew Chem-Int Edit 54:11073–11077. doi:10.1002/anie.201505232
Kim K, Lee HB, Lee JW, Park HK, Shin KS (2008) Self-assembly of poly(ethylenimine)-capped Au nanoparticles at a toluene-water interface for efficient surface-enhanced Raman scattering. Langmuir 24:7178–7183. doi:10.1021/la800733x
Zhang AQ, Cai LJ, Sui L, Qian DJ, Chen M (2013c) Reducing properties of polymers in the synthesis of noble metal nanoparticles. Polym Rev 53:240–276. doi:10.1080/15583724.2013.776587
Hvolbaek B, Janssens TVW, Clausen BS, Falsig H, Christensen CH, Norskov JK (2007) Catalytic activity of Au nanoparticles. Nano Today 2:14–18. doi:10.1016/s1748-0132(07)70113-5
Liu Y, Liu LL, Yuan M, Guo R (2013) Preparation and characterization of casein-stabilized gold nanoparticles for catalytic applications. Colloid Surf A-Physicochem Eng Asp 417:18–25. doi:10.1016/j.colsurfa.2012.08.050
Wu H, Huang X, Gao MM, Liao XP, Shi B (2011) Polyphenol-grafted collagen fiber as reductant and stabilizer for one-step synthesis of size-controlled gold nanoparticles and their catalytic application to 4-nitrophenol reduction. Green Chem 13:651–658. doi:10.1039/c0gc00843e
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21603196), the Natural Science Foundation of Hubei Province (No. 2015CFB697), the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (No. CUG170601), and the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing (Wuhan University of Technology).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, Y., Ren, T., Wang, Y. et al. The synergistic effect of nitrogen atoms and triblock structure on stabilizing gold nanoparticles for catalytic reduction of 4-nitrophenol. Gold Bull 50, 123–129 (2017). https://doi.org/10.1007/s13404-017-0204-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-017-0204-1