Abstract
Purpose
Tamoxifen is a major treatment modality for estrogen receptor positive breast cancer, but the occurrence of resistance remains a problem. Recently, obesity-related leptin has been found to interfere with tamoxifen in breast cancer MCF-7 cells. In the present study we investigated the effect of leptin on three tamoxifen-treated breast cancer cell types (i.e., MDA-MB-231, MCF-7 and MCF-7/HER2).
Methods
The effect of tamoxifen/leptin treatment was evaluated using a MTT cell viability assay. mRNA expression was assessed by real time PCR and protein expression by Western blotting. WWOX, Survivin and BCL2 gene promoter activities were evaluated by chromatin immunoprecipitation.
Results
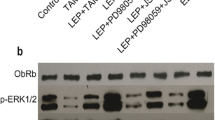
Cell viability assays revealed that estrogen receptor negative MDA-MB-231 cells were resistant, that estrogen receptor positive MCF-7 cells were sensitive and that MCF-7/HER2 cells were relatively resistant to tamoxifen, while leptin co-administration ‘rescued’ MCF-7 and, especially, MCF-7/HER2 cells from the anti-proliferative effect of tamoxifen. The cell lines also exhibited a different phosphorylation status of STAT3, a transcription factor that is activated by the obesity related leptin receptor b (Ob-Rb). Most importantly, chromatin immunoprecipitation assays revealed differential STAT3 binding to the anti-apoptotic BCL2 and pro-apoptotic WWOX gene promoters in MCF-7 and MCF-7/HER2 cells, leading to concomitant modifications of its mRNA/protein expression levels, thus providing a selective advantage to HER2 over-expressing MCF-7/HER2 cells after treatment with tamoxifen and tamoxifen plus leptin.
Conclusions
Our study provides novel evidence indicating that synergy between the leptin/Ob-Rb/STAT3 signalling pathway and the HER2 receptor protects tamoxifen-treated HER2 over-expressing cells from the inhibitory effect of tamoxifen through differential regulation of apoptosis-related genes.




Similar content being viewed by others
References
K. McPherson, C.M. Steel, J.M. Dixon, ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ 321, 624–628 (2000)
B. Pula, M. Olbromski, A. Wojnar, A. Gomulkiewicz, W. Witkiewicz, M. Ugorski, P. Dziegiel, M. Podhorska-Okolow, Impact of SOX18 expression in cancer cells and vessels on the outcome of invasive ductal breast carcinoma. Cell. Oncol. 36, 469–483 (2013)
J.L. Botha, F. Bray, R. Sankila, D.M. Parkin, Breast cancer incidence and mortality trends in 16 European countries. Eur. J. Cancer 39, 1718–1729 (2003)
J.S. de Groot, X. Pan, J. Meeldijk, E. van der Wall, P.J. van Diest, C.B. Moelans, Validation of DNA promoter hypermethylation biomarkers in breast cancer–a short report. Cell. Oncol. 37, 297–303 (2014)
S. Loi, B. Haibe-Kains, C. Desmedt, P. Wirapati, F. Lallemand, A.M. Tutt, C. Gillet, P. Ellis, K. Ryder, J.F. Reid, M.G. Daidone, M.A. Pierotti, E.M. Berns, M.P. Jansen, J.A. Foekens, M. Delorenzi, G. Bontempi, M.J. Piccart, C. Sotiriou, Predicting prognosis using molecular profiling in estrogen receptor-positive breast cancer treated with tamoxifen. BMC Genomics 9, 239 (2008)
A. Halon, P. Donizy, P. Surowiak, R. Matkowski, ERM/Rho protein expression in ductal breast cancer: a 15 year follow-up. Cell. Oncol. 36, 181–190 (2013)
S. Tabarestani, S.M. Ghaderian, H. Rezvani, R. Mirfakhraie, A. Ebrahimi, H. Attarian, J. Rafat, M. Ghadyani, H.A. Alavi, N. Kamalian, A. Rakhsha, E. Azargashb, Prognostic and predictive value of copy number alterations in invasive breast cancer as determined by multiplex ligation-dependent probe amplification. Cell. Oncol. 37, 107–118 (2014)
P.J. Goodwin, M. Ennis, I.G. Fantus, K.I. Pritchard, M.E. Trudeau, J. Koo, N. Hood, Is leptin a mediator of adverse prognostic effects of obesity in breast cancer? J. Clin. Oncol. 23, 6037–6042 (2005)
M. Sulkowska, J. Golaszewska, A. Wincewicz, M. Koda, M. Baltaziak, S. Sulkowski, Leptin–from regulation of fat metabolism to stimulation of breast cancer growth. Pathol. Oncol. Res. 12, 69–72 (2006)
E.E. Calle, C. Rodriguez, K. Walker-Thurmond, M.J. Thun, Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 348, 1625–1638 (2003)
D.P. Rose, E.M. Gilhooly, D.W. Nixon, Adverse effects of obesity on breast cancer prognosis, and the biological actions of leptin (review). Int. J. Oncol. 21, 1285–1292 (2002)
S.H. Kim, A. Nagalingam, N.K. Saxena, S.V. Singh, D. Sharma, Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis 32, 359–367 (2011)
Y. Zhang, R. Proenca, M. Maffei, M. Barone, L. Leopold, J.M. Friedman, Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994)
R.S. Ahima, J.S. Flier, Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 11, 327–332 (2000)
Y. Matsuzawa, Adipocytokines and metabolic syndrome. Semin. Vasc. Med. 5, 34–39 (2005)
G. Fruhbeck, Intracellular signalling pathways activated by leptin. Biochem. J. 393, 7–20 (2006)
S. Collins, C.M. Kuhn, A.E. Petro, A.G. Swick, B.A. Chrunyk, R.S. Surwit, Role of leptin in fat regulation. Nature 380, 677 (1996)
R.B. Harris, Leptin–much more than a satiety signal. Annu. Rev. Nutr. 20, 45–75 (2000)
M.C. Henson, V.D. Castracane, Leptin in pregnancy. Biol. Reprod. 63, 1219–1228 (2000)
F. Zhang, Y. Chen, M. Heiman, R. Dimarchi, Leptin: structure, function and biology. Vitam. Horm. 71, 345–372 (2005)
L.A. Tartaglia, M. Dembski, X. Weng, N. Deng, J. Culpepper, R. Devos, G.J. Richards, L.A. Campfield, F.T. Clark, J. Deeds, C. Muir, S. Sanker, A. Moriarty, K.J. Moore, J.S. Smutko, G.G. Mays, E.A. Wool, C.A. Monroe, R.I. Tepper, Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271 (1995)
X. Hu, S.C. Juneja, N.J. Maihle, M.P. Cleary, Leptin–a growth factor in normal and malignant breast cells and for normal mammary gland development. J. Natl. Cancer Inst. 94, 1704–1711 (2002)
K. Laud, I. Gourdou, L. Pessemesse, J.P. Peyrat, J. Djiane, Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol. Cell. Endocrinol. 188, 219–226 (2002)
D. Cirillo, A.M. Rachiglio, R. la Montagna, A. Giordano, N. Normanno, Leptin signaling in breast cancer: an overview. J. Cell. Biochem. 105, 956–964 (2008)
S.N. O’Brien, B.H. Welter, T.M. Price, Presence of leptin in breast cell lines and breast tumors. Biochem. Biophys. Res. Commun. 259, 695–698 (1999)
M. Ishikawa, J. Kitayama, H. Nagawa, Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin. Cancer Res. 10, 4325–4331 (2004)
C. Bjorbaek, S. Uotani, B. da Silva, J.S. Flier, Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 272, 32686–32695 (1997)
R. Devos, Y. Guisez, J. Van der Heyden, D.W. White, M. Kalai, M. Fountoulakis, G. Plaetinck, Ligand-independent dimerization of the extracellular domain of the leptin receptor and determination of the stoichiometry of leptin binding. J. Biol. Chem. 272, 18304–18310 (1997)
J.E. Darnell Jr., STATs and gene regulation. Science 277, 1630–1635 (1997)
Q. Gao, M.J. Wolfgang, S. Neschen, K. Morino, T.L. Horvath, G.I. Shulman, X.Y. Fu, Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl. Acad. Sci. U. S. A. 101, 4661–4666 (2004)
H. Inoue, W. Ogawa, M. Ozaki, S. Haga, M. Matsumoto, K. Furukawa, N. Hashimoto, Y. Kido, T. Mori, H. Sakaue, K. Teshigawara, S. Jin, H. Iguchi, R. Hiramatsu, D. LeRoith, K. Takeda, S. Akira, M. Kasuga, Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat. Med. 10, 168–174 (2004)
D.E. Levy, J.E. Darnell Jr., Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3, 651–662 (2002)
J. Abdulghani, L. Gu, A. Dagvadorj, J. Lutz, B. Leiby, G. Bonuccelli, M.P. Lisanti, T. Zellweger, K. Alanen, T. Mirtti, T. Visakorpi, L. Bubendorf, M.T. Nevalainen, Stat3 promotes metastatic progression of prostate cancer. Am. J. Pathol. 172, 1717–1728 (2008)
R. Catlett-Falcone, T.H. Landowski, M.M. Oshiro, J. Turkson, A. Levitzki, R. Savino, G. Ciliberto, L. Moscinski, J.L. Fernandez-Luna, G. Nunez, W.S. Dalton, R. Jove, Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10, 105–115 (1999)
Z. Duan, R. Foster, D.A. Bell, J. Mahoney, K. Wolak, A. Vaidya, C. Hampel, H. Lee, M.V. Seiden, Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin. Cancer Res. 12, 5055–5063 (2006)
T. Gritsko, A. Williams, J. Turkson, S. Kaneko, T. Bowman, M. Huang, S. Nam, I. Eweis, N. Diaz, D. Sullivan, S. Yoder, S. Enkemann, S. Eschrich, J.H. Lee, C.A. Beam, J. Cheng, S. Minton, C.A. Muro-Cacho, R. Jove, Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin. Cancer Res. 12, 11–19 (2006)
B.B. Aggarwal, G. Sethi, K.S. Ahn, S.K. Sandur, M.K. Pandey, A.B. Kunnumakkara, B. Sung, H. Ichikawa, Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann. N. Y. Acad. Sci. 1091, 151–169 (2006)
S. Fletcher, J. Turkson, P.T. Gunning, Molecular approaches towards the inhibition of the signal transducer and activator of transcription 3 (Stat3) protein. ChemMedChem 3, 1159–1168 (2008)
E.B.C.T.C. Group, Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 351, 1451–1467 (1998)
E. Fiorio, A. Mercanti, M. Terrasi, R. Micciolo, A. Remo, A. Auriemma, A. Molino, V. Parolin, B. Di Stefano, F. Bonetti, A. Giordano, G.L. Cetto, E. Surmacz, Leptin/HER2 crosstalk in breast cancer: in vitro study and preliminary in vivo analysis. BMC Cancer 8, 305 (2008)
X. Chen, X. Zha, W. Chen, T. Zhu, J. Qiu, O.D. Roe, J. Li, Z. Wang, Y. Yin, Leptin attenuates the anti-estrogen effect of tamoxifen in breast cancer. Biomed. Pharmacother. 67, 22–30 (2012)
M. Harvie, L. Hooper, A.H. Howell, Central obesity and breast cancer risk: a systematic review. Obes. Rev. 4, 157–173 (2003)
P.H. Lahmann, K. Hoffmann, N. Allen, C.H. van Gils, K.T. Khaw, B. Tehard, F. Berrino, A. Tjonneland, J. Bigaard, A. Olsen, K. Overvad, F. Clavel-Chapelon, G. Nagel, H. Boeing, D. Trichopoulos, G. Economou, G. Bellos, D. Palli, R. Tumino, S. Panico, C. Sacerdote, V. Krogh, P.H. Peeters, H.B. Bueno-de-Mesquita, E. Lund, E. Ardanaz, P. Amiano, G. Pera, J.R. Quiros, C. Martinez, M.J. Tormo, E. Wirfalt, G. Berglund, G. Hallmans, T.J. Key, G. Reeves, S. Bingham, T. Norat, C. Biessy, R. Kaaks, E. Riboli, Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC). Int. J. Cancer 111, 762–771 (2004)
K.B. Michels, K.L. Terry, W.C. Willett, Longitudinal study on the role of body size in premenopausal breast cancer. Arch. Intern. Med. 166, 2395–2402 (2006)
R.V. Considine, M.K. Sinha, M.L. Heiman, A. Kriauciunas, T.W. Stephens, M.R. Nyce, J.P. Ohannesian, C.C. Marco, L.J. McKee, T.L. Bauer et al., Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996)
M. Maffei, J. Halaas, E. Ravussin, R.E. Pratley, G.H. Lee, Y. Zhang, H. Fei, S. Kim, R. Lallone, S. Ranganathan et al., Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1, 1155–1161 (1995)
S. Catalano, S. Marsico, C. Giordano, L. Mauro, P. Rizza, M.L. Panno, S. Ando, Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J. Biol. Chem. 278, 28668–28676 (2003)
S. Catalano, L. Mauro, S. Marsico, C. Giordano, P. Rizza, V. Rago, D. Montanaro, M. Maggiolini, M.L. Panno, S. Ando, Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J. Biol. Chem. 279, 19908–19915 (2004)
C. Garofalo, D. Sisci, E. Surmacz, Leptin interferes with the effects of the antiestrogen ICI 182,780 in MCF-7 breast cancer cells. Clin. Cancer Res. 10, 6466–6475 (2004)
A. Valle, J. Sastre-Serra, J. Oliver, P. Roca, Chronic leptin treatment sensitizes MCF-7 breast cancer cells to estrogen. Cell Physiol. Biochem. 28, 823–32 (2011)
C. Carlomagno, F. Perrone, C. Gallo, M. De Laurentiis, R. Lauria, A. Morabito, G. Pettinato, L. Panico, A. D’Antonio, A.R. Bianco, S. De, Placido, c-erb B2 overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. J. Clin. Oncol. 14, 2702–8 (1996)
C.K. Osborne, V. Bardou, T.A. Hopp, G.C. Chamness, S.G. Hilsenbeck, S.A. Fuqua, J. Wong, D.C. Allred, G.M. Clark, R. Schiff, Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl. Cancer Inst. 95, 353–361 (2003)
I.E. Smith, M. Dowsett, S.R. Ebbs, J.M. Dixon, A. Skene, J.U. Blohmer, S.E. Ashley, S. Francis, I. Boeddinghaus, G. Walsh, Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J. Clin. Oncol. 23, 5108–5116 (2005)
J. Shou, S. Massarweh, C.K. Osborne, A.E. Wakeling, S. Ali, H. Weiss, R. Schiff, Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J. Natl. Cancer Inst. 96, 926–935 (2004)
C. Giordano, D. Vizza, S. Panza, I. Barone, D. Bonofiglio, M. Lanzino, D. Sisci, F. De Amicis, S.A. Fuqua, S. Catalano, S. Ando, Leptin increases HER2 protein levels through a STAT3-mediated up-regulation of Hsp90 in breast cancer cells. Mol. Oncol. 7, 379–391 (2013)
P.K. Epling-Burnette, J.H. Liu, R. Catlett-Falcone, J. Turkson, M. Oshiro, R. Kothapalli, Y. Li, J.M. Wang, H.F. Yang-Yen, J. Karras, R. Jove, T.P. Loughran Jr., Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Invest. 107, 351–362 (2001)
S. Alas, B. Bonavida, Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B-non-Hodgkin’s lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in down-regulation of Bcl-2 and sensitization to cytotoxic drugs. Cancer Res. 61, 5137–5144 (2001)
S. Alas, B. Bonavida, Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin’s lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin. Cancer Res. 9, 316–326 (2003)
N. Diaz, S. Minton, C. Cox, T. Bowman, T. Gritsko, R. Garcia, I. Eweis, M. Wloch, S. Livingston, E. Seijo, A. Cantor, J.H. Lee, C.A. Beam, D. Sullivan, R. Jove, C.A. Muro-Cacho, Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin. Cancer Res. 12, 20–28 (2006)
D.C. Altieri, Validating survivin as a cancer therapeutic target. Nat. Rev. Cancer 3, 46–54 (2003)
A.K. Bednarek, C.L. Keck-Waggoner, R.L. Daniel, K.J. Laflin, P.L. Bergsagel, K. Kiguchi, A.J. Brenner, C.M. Aldaz, WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res. 61, 8068–8073 (2001)
N.S. Chang, J. Doherty, A. Ensign, JNK1 physically interacts with WW domain-containing oxidoreductase (WOX1) and inhibits WOX1-mediated apoptosis. J. Biol. Chem. 278, 9195–9202 (2003)
N.S. Chang, N. Pratt, J. Heath, L. Schultz, D. Sleve, G.B. Carey, N. Zevotek, Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J. Biol. Chem. 276, 3361–3370 (2001)
G. Guler, A. Uner, N. Guler, S.Y. Han, D. Iliopoulos, P. McCue, K. Huebner, Concordant loss of fragile gene expression early in breast cancer development. Pathol. Int. 55, 471–478 (2005)
M.I. Nunez, J. Ludes-Meyers, M.C. Abba, H. Kil, N.W. Abbey, R.E. Page, A. Sahin, A.J. Klein-Szanto, C.M. Aldaz, Frequent loss of WWOX expression in breast cancer: correlation with estrogen receptor status. Breast Cancer Res. Treat. 89, 99–105 (2005)
R.I. Aqeilan, Y. Pekarsky, J.J. Herrero, A. Palamarchuk, J. Letofsky, T. Druck, F. Trapasso, S.Y. Han, G. Melino, K. Huebner, C.M. Croce, Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc. Natl. Acad. Sci. U. S. A. 101, 4401–4406 (2004)
R.I. Aqeilan, A. Palamarchuk, R.J. Weigel, J.J. Herrero, Y. Pekarsky, C.M. Croce, Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res. 64, 8256–8261 (2004)
E. Gaudio, A. Palamarchuk, T. Palumbo, F. Trapasso, Y. Pekarsky, C.M. Croce, R.I. Aqeilan, Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res. 66, 11585–11589 (2006)
N.S. Chang, L.J. Hsu, Y.S. Lin, F.J. Lai, H.M. Sheu, WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol. Med. 13, 12–22 (2007)
D. Trivigno, F. Essmann, S.M. Huber, J. Rudner, Deubiquitinase USP9x confers radioresistance through stabilization of Mcl-1. Neoplasia, 14, 893–904 (2012)
J. Zhao, T. Tenev, L.M. Martins, J. Downward, N.R. Lemoine, The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J. Cell Sci. 113(Pt 23), 4363–4371 (2000)
V. Papanikolaou, D. Iliopoulos, I. Dimou, S. Dubos, C. Kappas, S. Kitsiou-Tzeli, A. Tsezou, Survivin regulation by HER2 through NF-kappaB and c-myc in irradiated breast cancer cells. J. Cell. Mol. Med. 15, 1542–1550 (2011)
Conflict of interest
The authors have no conflict of interest to report with respect to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papanikolaou, V., Stefanou, N., Dubos, S. et al. Synergy of leptin/STAT3 with HER2 receptor induces tamoxifen resistance in breast cancer cells through regulation of apoptosis-related genes. Cell Oncol. 38, 155–164 (2015). https://doi.org/10.1007/s13402-014-0213-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-014-0213-5