Abstract
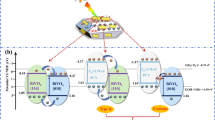
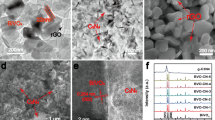
Reducing the fast recombination of photogenerated electron-hole pairs of semiconductor photocatalyst is very important to improve its photocatalysis. In this paper we fabricate Cu2O nanocube-decorated BiVO4 nanocrystal (denoted as BiVO4@Cu2O nanocrystal@nanocube) heterostructure photocatalyst by coupling n-type BiVO4 with p-type Cu2O. The BiVO4@Cu2O nanocrystal@nanocube photocatalysts show superior photocatalytic activities in photoelectrochemical (PEC) activity and photocatalytic water oxidation to BiVO4 photocatalysts under visible light illumination. The BiVO4@Cu2O nanocrystal@nanocube heterostructure electrode achieves the highest photocurrent density of ∼ 10 μA cm−2 at 0 V versus Ag/AgCl, 5 times higher than that of BiVO4 nanocrystal electrode (∼ 2 μA cm−2). The light induced evolution rate of O2 generation for BiVO4@Cu2O nanocrystal@nanocube heterostructures is as high as 150 μmol h−1100 mg cat−1, more than 3 times higher than that (48 μmol h−1100 mg cat−1) of BiVO4 nanocrystals. The enhanced photocatalysis activities of the BiVO4@Cu2O nanocrystal@nanocube photocatalysts are attributed to the efficient separation of the photoexcited electron-hole pairs caused by inner electronic field (IEF) of p-n junction. This study opens up new opportunities in designing photoactive materials with highly enhanced performance for solar energy conversion.

Similar content being viewed by others
References
X. Chen, S. Shen, L. Guo, and S. S. Mao, Chem. Rev. 111, 6503 (2010).
A. Paracchino, V. Laporte, K. Sivula, M. Gratzel, and E. Thimsen, Nat. Mater. 10, 456 (2011).
M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori, and N. S. Lewis, Chem. Rev. 111, 6446 (2010).
H. S. Kim, K.-S. Ahn, and S. H. Kang, Electron. Mater. Lett. 10, 345 (2014).
Y. J. Lin, S. Zhou, S. W. Sheehan, and D. W. Wang, J. Am. Chem. Soc. 133, 2398 (2011).
A. Yamaguchi, R. Inuzuka, T. Takashima, T. Hayashi, K. Hashimoto, and R. Nakamura, Nat. Commun. 5, 4256 (2014).
Y. H. Pu, G. M. Wang, K. D. Chang, Y. C. Ling, Y. K. Lin, B. C. Fitzmorris, C. M. Liu, X. H. Lu, Y. X. Tong, J. Z. Zhang, Y. J. Hsu, and Y. Li, Nano Lett. 13, 3817 (2013).
X. F. Zhang, Y. Gong, X. L. Dong, X. X. Zhang, C. Ma, and F. Shi, Mater. Chem. Phys. 136, 472 (2012).
Y. H. Ng, A. Iwase, A. Kudo, and R. Amal, J. Phys. Chem. Lett. 1, 2607 (2010).
A. L. Linsebigler, G. Lu, and J. T. Yates, Chem. Rev. 95, 735 (1995).
D. B. Ingram and S. Linic, J. Am. Chem. Soc. 133, 5202 (2011).
Y. Lin, Y. Xu, M. T. Mayer, Z. I. Simpson, G. McMahon, S. Zhou, and D. Wang, J. Am. Chem. Soc. 134, 5508 (2012).
S. Mandati, B. V. Sarada, S. R. Dey, and S. V. Joshi, Electron. Mater. Lett. 11, 618 (2015).
J. T. Li, F. K. Meng, S. Suri, W. Q. Ding, F. Q. Huang, and N. Q. Wu, Chem. Commun. 48, 8213 (2012).
M. Long, W. M. Cai, and H. Kisch, J. Phys. Chem. C 112, 548 (2008).
Y. X. Yu, W. X. Ouyang, Z. T. Liao, B. B. Du, and W. D. Zhang, ACS Appl. Mater. Interfaces 6, 8467 (2014).
Y. Park, K. J. McDonald, and K. S. Choi, Chem. Soc. Rev. 42, 2321 (2013).
T. W. Kim and K. S. Choi, Science 343, 990 (2014).
A. Kudo, K. Omori, and H. Kato, J. Am. Chem. Soc. 121, 11459 (1999).
F. F. Abdi and R. van de Krol, J. Phys. Chem. C 116, 9398 (2012).
D. K. Zhong, S. Choi, and D. R. Gamelin, J. Am. Chem. Soc. 133, 18370 (2011).
C. K. Wu, M. Yin, S. O’Brien, and J. T. Koberstein, Chem. Mater. 18, 6054 (2006).
C. D. Wagner, W. M. Riggs, L. E. Davis, J. E. Moulder, and G. E. Muilenber, Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corporation Physical Electronics Division, USA (1979).
H. Q. Jiang, H. Endo, H. Natori, M. Nagai, and K. Kobayashi, Mater. Res. Bull. 44, 700 (2009).
Y. Ohko, K. Hashimoto, and A. Fujishima, J. Phys. Chem. A 101, 8057 (1997).
Y. M. He, J. Cai, T. T. Li, Y. Wu, H. J. Lin, L. H. Zhao, and M. F. Luo, Chem. Eng. J. 215-216, 721 (2013).
X. Nie, J. Y. Chen, G. Y. Li, H. X. Shi, H. J. Zhao, P. K. Wong, and T. C. An, J. Chem. Technol. Biotechnol. 88, 1488 (2013).
P. He, X. Shen, and H. Gao, J. Colloids Interface Sci. 284, 510 (2005).
P. Chatchai, Y. Murakami, S. Y. Nosaka, A. Y. Kishioka, and Y. Nosaka, Electrochim. Acta 54, 1147 (2009).
H. Xu, H. Li, C. Wu, J. Chu, Y. Yan, H. Shu, and Z. Gu, J. Hazard. Mater. 153, 877 (2008).
W. Y. Yang and S. W. Rhee, Appl. Phys. Lett. 91, 232907 (2007).
M. C. Long, W. M. Cai, J. Cai, B. X. Zhou, X. Y. Chai, and Y. H. Wu, J. Phys. Chem. B 110, 20211 (2006).
V. Subramanian, E. E. Wolf, and P. V. Kamat, J. Am. Chem. Soc. 126, 4943 (2004).
J. W. Tang, J. R. Durrant, and D. R. Klug, J. Am. Chem. Soc. 130, 13885 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Zhang, W., Meng, S. et al. Enhanced photoelectrochemical water splitting and photocatalytic water oxidation of Cu2O nanocube-loaded BiVO4 nanocrystal heterostructures. Electron. Mater. Lett. 12, 753–760 (2016). https://doi.org/10.1007/s13391-016-6224-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-016-6224-9