Abstract
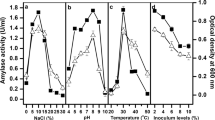
This work pivots around screening a toluene-tolerant bacterium with concomitant \(\upalpha \)-amylase production ability. Eighteen \(\upalpha \)-amylase-producing bacterial strains were isolated from samples collected from Tokat province of Turkey among which strain A7 showed maximum \(\upalpha \)-amylase production titers (968 U/ml). The strain A7 was identified as Enterococcus faecalis mercadA7 (KX298857) with 54% GC content and 98% similarity to the closest strain, Enterococcus faecalis NBRC100480 through 16srRNA typing studies. The amylase production was improved 2.6-folds compared to the basal medium at optimal conditions recorded: \(37\,{^{\circ }}\)C, pH 7.0, 18 h incubation, 1%v/v inoculum content and 20% toluene (carbon) supplementation. The amylase activity was demonstrated to increase upon exposure to toluene by a yet to be determined mechanism. The various growth kinetic parameters recorded are specific growth rate, \(\mu _{\mathrm{max}}\) of 0.318 \(\hbox {h}^{-1}\); constant, \(K_{{s}}\) 10.48 mg/ml; yield coefficient, \(Y_{{x}/{s}}\) of 0.1157 mg/g; and cell doubling time, \(t_{\mathrm{d}}\) of 51 min. The enzyme was partially purified with a threefold increase in specific activity; it was found to be active at pH 8 and \(40\,{^{\circ }}\)C; stable from 30 to \(50\,{^{\circ }}\)C and 7–10 pH range; maintaining stability up to 40% of toluene. The purified enzyme’s maximum velocity, \(V_{\mathrm{max}}\) of 3.33 \(\hbox {mmol}/\hbox {h}\,\hbox {ml}\) with constant, Km of 5 mg/ml were determined by Line Weaver Burke plot. Both Enterococcus faecalis mercadA7 and the purified \(\upalpha \)-amylase might find applications in several industrial processes due to the catalytic promiscuity triggered by the presence of toluene, and augmented stability of the cell-free purified enzyme as well as the organism to high alkaline/toluene levels.
Similar content being viewed by others
References
Kathiresan, K.; Manivannan, S.: \(\upalpha \)-Amylase production by Penicillium fellutanum isolated from mangrove rhizosphere soil. Afr. J. Biotech. 5(10), 829–832 (2006)
Rajagopalan, G.; Krishnan, C.: \(\upalpha \)-Amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Biores. Technol. 99(8), 3044–3050 (2008)
Reddy, N.S.; Nimmagadda, A.; Rao, K.R.S.S.: An overview of the microbial \(\upalpha \)-amylase family. Afr. J. Biotech. 2(12), 645–648 (2003)
Pandey, A.; Nigam, P.; Soccol, C.R.; Soccol, V.T.; Singh, D.; Mohan, R.: Advances in microbial amylases. Biotechnol. Appl. Biochem. 31, 135–152 (2000)
Adebiyi, A.O.; Adebiyi, A.P.; Olaniyi, E.O.: Biological studies on albino rats fed with Sorghum bicolor starch hydrolyzed with \(\upalpha \)-amylase from Rhizopus sp. Afr. J. Biotechnol. 4, 1089–1094 (2005)
Omemu, A.M.; Akpan, I.; Bankole, M.O.; Teniola, O.D.: Hydrolysis of raw tuber starches by amylase of Aspergillus niger AM07 isolated from the soil. Afr. J. Biotechnol. 4, 19–25 (2005)
Aiyer, P.V.: Amylases and their applications. Afr. J. Biotechnol. 4(13), 1525–1529 (2005)
Thumar, J.T.; Singh, S.P.: Organic solvent tolerance of an alkaline protease from salt-tolerant alkaliphilic Streptomyces clavuligerus strain Mit-1. J. Ind. Microbiol. Biotechnol. 36, 211–218 (2009)
Shafiei, M.; Ziaee, A.A.; Amoozegar, M.A.: Purification and characterization of an organic-solvent-tolerant halophilic \(\upalpha \)-amylase from the moderately halophilic Nesterenkonia sp. strain F. J. Ind. Microbiol. Biotechnol. 38, 275–281 (2011)
Mrowiec, B.: Toluene in sewage and sludge in wastewater treatment plants. Water Sci. Technol. 69(1), 128–134 (2014)
Sikkema, J.; De Bont, J.A.M.; Poolman, B.: Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 269, 8022–8028 (1994)
Ogino, H.; Ishikawa, H.: Enzymes which are stable in the presence of organic solvents. J. Biosci. Bioeng. 91, 109–116 (2001)
Madigan, M.T.; Martinko, J.M.; Stahl, D.A.; Clarck, D.P.: Brock Biology of Microorganisms, 13th edn, pp. 642–656. Prentice Hall, Englewood Cliffs (2011)
Yoo, Y.J.; Hong, J.; Hatch, R.T.: Comparison of \(\upalpha \)-amylase activities from different assay methods. Biotechnol. Bioeng. 30, 147–151 (1987)
Creig, R.N.; Holt, G.J.: Bergey’s Manual of Systematic Bacteriology. Williams & Wilkins, London (1984)
Yadav, V.; Prakash, S.; Srivastava, S.; Verma, P.C.; Gupta, V.; Basu, V.; Rawat, A.K.: Identification of Comamonas species using 16S rRNA gene sequence. J. Bioinform. 3, 381–383 (2009)
Abd-Elhalem, B.T.; El-Sawy, M.; Gamal, R.F.; Abou-Taleb, K.A.: Production of amylases from Bacillus amyloliquefaciens under submerged fermentation using some agro-industrial by-products. Ann. Agric. Sci. 60(2), 193–202 (2015)
Thompson, J.D.; Higgins, D.G.; Gibson, T.J.; Clustal, W.: Improving the sensitivity of progressive multiple sequence alignment though sequence weighting, position-specific gap penalties and weight matrix choice. Nucl. Acids Res. 22, 4673–4680 (1994)
Altschuf, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J.: Grapped BLAST and PSI BLAST: a new generation of protein database search programs. Nucl. Acid 25, 3389–3402 (1997)
Saitou, N.; Nei, M.: The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4(4), 406–425 (1987)
Monod, J.: The growth of bacterial cultures. Ann. Rev. Microbiol. 3, 371–394 (1949)
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970)
Bernfeld, P.: Amylases \(\upalpha \) and beta. In: Colowick, S.P., Kaplan, N.O. (eds.) Methods in Enzymology, vol. 1, pp. 149–158. Academic Press, New York (1995)
Ibrahim, A.N.; Ahmed, F.H.; Ibrahim, M.M.K.; Arafa, M.A.I.: Precipitation and purification of amylase enzyme produced by Streptomyces aureofaciens 77. Arch. Pharm. Res. 13(1), 28–32 (1990)
Demirkan, E.: Production, purification, and characterization of \(\upalpha \)-amylase by Bacillus subtilis and its mutant derivates. Turk. J. Biol. 35, 705–712 (2011)
Solheim, M.; Aakra, Å.; Snipen, L.G.; Brede, D.A.; Nes, I.F.: Comparative genomics of Enterococcus faecalis from healthy Norwegian infants. BMC Genomics 10, 194 (2009)
Deb, P.; Talukdar, S.A.; Mohsina, K.; Sarker, P.K.; Abu Sayem, S.M.: Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. SpringerPlus 2, 154 (2013)
Raplong, H.H.; Odeleye, P.O.; Idoko, M.O.; Asanato, J.I.; Odeke, E.H.: Production of \(\upalpha \) amylase by Bacillus cereus in submerged fermentation. Aceh Int. J. Sci. Technol. 3, 124–130 (2014)
Ahmed, K.; Valeem, E.E.; Khan, M.A.; Qamar-ul-Haq, : Biosynthesis of \(\upalpha \) amylase from Aspergillus fumigatus (fresenius 1863) in submerged fermentation. Pak. J. Biotechnol. 12(2), 87–92 (2015)
Yoon, M.Y.; Yoo, Y.J.; Cadman, T.W.: Phosphate effects in the fermentation of \(\upalpha \)-amylase by Bacillus amyloliquefaciens. Biotechnol. Lett. 11(1), 57–60 (1989)
Rukhaiyar, R.; Srivastav, S.K.: Effect of various carbon substrates on \(\upalpha \)-amylase production from Bacillus species. J. Microbiol. Biotechnol. 10, 76–82 (1995)
Alghabpoor, S.S.; Panosyan, H.; Popov, Y.: Production of thermostable \(\upalpha \)-amylase by Bacillus sp. Iranian s2 using solid state fermentation. Electron. J Nat. Sci. 2(21), 47–50 (2013)
Mukhtar, H.; Ikram-Ul-Haq, : Concomitant production of two proteases and \(\upalpha \)-amylase by a novel strain of Bacillus subtilis in a microprocessor controlled bioreactor. Braz. J. Microbiol. 43, 1072–1079 (2012)
Hillier, P.; Wase, D.A.J.; Emery, A.N.; Solomons, G.L.: Instability of amylase production and morphological variation in continuous culture of Bacillus amyloliquefaciens is associated with plasmid loss. Process Biochem. 32, 51–59 (1997)
Lonsane, B.K.; Ghildyal, N.P.; Budiatman, S.; Ramakrishna, S.V.: Engineering aspects of solid state fermentation. Enzyme Microb. Technol. 7, 258–265 (1985)
Emenike, O.B.; Chibuzo, C.F.; Sabinus, O.O.E.: Characterization of partially purified-amylase from germinating African breadfruit (Treculia africana) seeds. Int. J. Pharm. Med. Sci. 5(1), 15–21 (2015)
Behal, A.; Singh, J.; Sharma, M.K.; Puri, P.; Batra, N.: Characterization of alkaline \(\upalpha \)-amylase from Bacillus sp. AB 04. Braz. J. Microbiol. 41(4), 850–861 (2010)
Syed, D.G.; Agasar, D.; Pandey, A.: Production and partial purification of \(\upalpha \)-amylase from a novel isolate Streptomyces gulbargensis. J. Indus. Microbiol. Biotechnol. 36(2), 189–194 (2009)
Chakraborty, S.; Khopade, A.; Kokare, C.; Mahadik, K.; Chopade, B.: Isolation and characterization of novel \(\upalpha \)-amylase from marine Streptomyces sp. D1. J. Mol. Catal. B Enzym. 58, 17–23 (2009)
Sani, I.; Abdulhamid, A.; Bello, F.; Yahaya, M.; Bagudo, A.I.: Isolation, partial purification and characterization of \(\upalpha \)-amylase from Bacillus subtilis. J. Microbiol. Biotechnol. Res. 4, 49–54 (2014)
Englard, S.; Seifter, S.: Precipitation techniques. In: Murray, E.D.; Dentscher, P. (eds.) Methods in Enzymology, vol. 182, pp. 425–441. Elsevier, USA (1990)
Spencer-Martin, I.: Extracellular isoamylase produced by the yeast Lipomyces kononenkae. Eur. J. Appl. Microbiol. 44(60), 1253–1257 (1982)
de Souza, P.M.; de Oliveira, M.P.: Application of microbial \(\upalpha \)-amylase in industry: a review. Braz. J. Microbiol. 41(4), 850–61 (2010)
Kumar, S.; Khare, S.K.: Purification and characterization of maltooligosaccharide-forming \(\upalpha \)-amylase from moderately halophilic Marinobacter sp. EMB8. Bioresour. Technol. 116, 247–251 (2012)
Tiwari, S.; Shukla, N.; Mishra, P.; Gaur, R.: Enhanced production and characterization of a solvent stable amylase from solvent tolerant Bacillus tequilensis RG-01: thermostable and surfactant resistant. Sci. World J. Article ID 972763, 1–11 (2014)
Segura, A.; Hurtado, A.; Rivera, B.; Lazaroaie, M.M.: Isolation of new toluene-tolerant marine strains of bacteria and characterization of their solvent-tolerance properties. J. Appl. Microbiol. 104(5), 1408–1416 (2008)
Li, X.; Yu, H.Y.: Characterization of an organic solvent-tolerant \(\upalpha \)-amylase from a halophilic isolate Thalassobacillus sp. LY18. Folia Microbiol. 57(5), 447–453 (2012)
Chang, J.; Lee, Y.S.; Fang, S.J.: Recombinant expression and characterization of an organic-solvent-tolerant \(\upalpha \)-amylase from Exiguobacterium sp. DAU5. Appl. Biochem. Biotechnol. 169, 1870–1883 (2013)
Moshfegh, M.; Shahverdi, A.R.; Zarrini, G.: Biochemical characterization of an extracellular polyextremophilic \(\upalpha \)-amylase from the halophilic archaeon Halorubrum xinjiangense. Extremophiles 17, 677–687 (2013)
Fukushima, T.; Mizuki, T.; Echigo, A.; Inoue, A.; Usami, R.: Organic solvent tolerance of halophilic \(\upalpha \)-amylase from a Haloarchaeon, Haloarcula sp. strain S-1. Extremophiles 9(1), 85–89 (2005)
Pandey, S.; Singh, S.P.: Organic solvent tolerance of an [\(\upalpha \)]-amylase from haloalkaliphilic bacteria as a function of pH, temperature, and salt concentrations. Appl. Biochem. Biotechnol. 166(7), 1747–1757 (2012)
Hossain, S.M.Z.; Haki, G.D.; Rakshit, S.K.: Optimum production and characterization of thermostable amylolytic enzymes from B. stearothermophilus GRE1. Can. J. Chem. Eng. 84, 368–374 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meruvu, H. Catalytic Profile and Amylolytic Studies of Toluene-Tolerant Enterococcus faecalis str. nov. mercadA7. Arab J Sci Eng 42, 1517–1527 (2017). https://doi.org/10.1007/s13369-016-2386-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-016-2386-x