Abstract
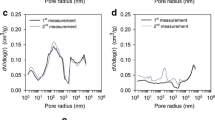
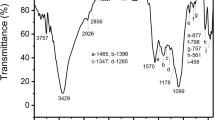
The Kingdom of Saudi Arabia is one of the leading countries in date fruit farming, and by-products from date palm trees can be used for several important applications, including pollution control. This study successfully employed date palm pit-based granular activated carbon (GAC) to adsorb gas-phase benzene under dynamic flow conditions. The percent carbon content (w/w) for raw date palm pits and produced GAC samples was found to be 47 and 82 %, respectively. Furthermore, the specific surface area (SSABET) of the produced GAC was 822 m2/g, and the t-Plot micropore area and t-Plot external surface area were 734.99 and 87.26 m2/g, respectively. The BJH graph for the pore size distribution also indicated a mesoporous structure. The use of date palm pit-based GAC for gas-phase benzene adsorption under dynamic continuous-flow conditions showed high efficiency with breakthrough points for different systems ranging from several hours to several days. The role of surface functional groups and their interactions with the benzene rings during the adsorption process were also explored, and surface oxygen-based groups may initiate an electron donor–acceptor mechanism with the benzene’s aromatic ring \({{\boldsymbol{\pi}}}\) electrons. The findings confirmed that GAC produced from date palm pits can be successfully used for gas-phase benzene adsorption under various conditions. It is hoped that countries with large-scale date fruit farming, such as the Kingdom of Saudi Arabia, will be able to utilize this rich resource for environmental applications.
Similar content being viewed by others
Abbreviations
- GAC:
-
Granular activated carbon
- SSABET :
-
BET specific surface area
References
Long C.; Li Y.; Yu W.; Li A.: Removal of benzene and methyl ethyl ketone vapor: comparison of hypercrosslinked polymeric adsorbent with activated carbon. J. Hazard Mater. 203(204), 251–256 (2012)
Kim K.-J.; Kang C.-S.; You Y.-J.; Chung M.-C.; Woo M.-W.; Jeong W.-J.; Park N.-C.; Ahn H.-G.: Adsorption–desorption characteristics of VOCs over impregnated activated carbons. Catal. Today 111, 223–228 (2006)
Metcalf & Eddy: Wastewater Engineering: Treatment and Reuse. McGraw-Hill, New York (2003)
Simonich S.L.; Begley W.M.; Debaere G.; Eckhoff W.S.: Trace analysis of fragrance materials in wastewater and treated wastewater. Environ. Sci. Technol. 34, 959–965 (2000)
Shin H.-C.; Park J.-W.; Park K.; Song H.-C.: Removal characteristics of trace compounds of landfill gas by activated carbon adsorption. Environ. Pollut. 119, 227–236 (2002)
Borgie M.; Garat A.; Cazier F.; Delbende A.; Allorge D.; Ledoux F.; Courcot D.; Shirali P.; Dagher Z.: Traffic related air pollution. A pilot exposure assessment in Beirut, Lebanon. Chemosphere 96, 122–128 (2014)
Jo W.-K.; Yang C.-H.: Granular-activated carbon adsorption followed by annular-type photocatalytic system for control of indoor aromatic compounds. Sep. Purif. Technol. 66, 438–442 (2009)
Ondarts M.; Hort C.; Sochard S.; Platel V.; Moynault L.; Seby F.: Evaluation of compost and a mixture of compost and activated carbon as biofilter media for the treatment of indoor air pollution. Environ. Technol. 33, 273–284 (2012)
Tham K.W.; Zuraimi M.S.; Sekhar S.C.: Emission modelling and validation of VOCs’ source strengths in air-conditioned office premises. Environ. Int. 30, 1075–1088 (2004)
Wu B.-Z.; Feng T.-Z.; Sree U.; Chiu K.-H.; Lo J.-G.: Sampling and analysis of volatile organics emitted from wastewater treatment plant and drain system of an industrial science park. Anal. Chim. Acta 576, 100–111 (2006)
Atasoy E.; Dogeroglu T.; Kara S.: The estimation of NMVOC emissions from an urban-scale wastewater treatment plant. Water Res. 38, 3265–3274 (2004)
Nikolaou A.D.; Golfinopoulos S.K.; Kostopoulou M.N.; Kolokythas G.A.; Lekkas T.D.: Determination of volatile organic compounds in surface waters and treated wastewater in Greece. Water Res. 36, 2883–2890 (2002)
Escalas A.; Guadayol J.M.; Cortina M.; Rivera J.; Caixach J.: Time and space patterns of volatile organic compounds in a sewage treatment plant. Water Res. 37, 3913–3920 (2003)
Khan F.I.; Ghoshal A.K.: Removal of volatile organic compounds from polluted air. J. Loss Prev. Process Ind. 13, 527–545 (2000)
Lorimier C.; Subrenat A.; Coq L.L.; Cloirec P.L.: Adsorption of toluene onto activated carbon fibre cloths and felts: application to indoor air treatment. Environ. Technol. 26, 1217–1230 (2005)
Moussavi G.; Rashidi R.; Khavanin A.: The efficacy of GAC/MgO composite for destructive adsorption of benzene from waste air stream. Chem. Eng. J. 228, 741–747 (2013)
Veksha A.; Sasaoka E.; Uddin M.A.: The influence of porosity and surface oxygen groups of peat-based activated carbons on benzene adsorption from dry and humid air. Carbon 47, 2371–2378 (2009)
Gil R.R.; Ruiz B.; Lozano M.S.; Martínc M.J.; Fuente E.: VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem. Eng. J. 245, 80–88 (2014)
Fadhil A.B.; Deyab M.M.: Conversion of some fruit stones and shells into activated carbons. Arab. J. Sci. Eng. 33, 175–184 (2008)
Nor N.M.; Chung L.L.; Teong L.K.; Mohamed A.R.: Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—a review. J. Environ. Chem. Eng. 1, 658–666 (2013)
Dias J.M.; Alvim-Ferraz M.C.M.; Almeida M.F.; Rivera-Utrilla J.; Sánchez-Polo M.: Waste materials for activated carbon preparation and its use in aqueous-phase treatment: a review. J. Environ. Manag. 85, 833–846 (2007)
Daifullah A.A.M.; Girgis B.S.: Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloids Surf. A 214, 181–193 (2003)
Demirba A.: Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. J. Hazard Mater. 167, 1–9 (2009)
Alhamed Y.A.: Adsorption kinetics and performance of packed bed adsorber for phenol removal using activated carbon from dates’ stones. J. Hazard Mater. 170, 763–770 (2009)
Abdulkarim M.; Abu Al-Rub F.: Adsorption of lead ions from aqueous solution onto activated carbon and chemically-modified activated carbon prepared from date pits. Adsorp. Sci. Technol. 22, 119–134 (2004)
Al-Muhtaseb S.A.; El-Naas M.H.; Abdallah S.: Removal of aluminum from aqueous solutions by adsorption on date-pit and BDH activated carbons. J. Hazard Mater. 158, 300–307 (2008)
Awwad N.S.; Daifuallah A.A.M.; Al M.M.S.: Removal of Pb2+, Cd2+, Fe3+, and Sr2+ from aqueous solution by selected activated carbons derived from date pits. Solvent Extr. Ion Exch. 26, 764–782 (2008)
Banat F.; Al-Asheh S.; Al-Makhadmeh L.: Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem. 39, 193–202 (2003)
Belhachemi M.; Rios R.V.R.A.; Addoun F.; Silvestre-Albero J.; Sepúlveda-Escribano A.; Rodryíguez-Reinoso F.: Preparation of activated carbon from date pits: effect of the activation agent and liquid phase oxidation. J. Anal. Appl. Pyrolysis 86, 168–172 (2009)
Bouchelta C.; Medjram M.S.; Bertrand O.; Bellat J.P.: Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis 82, 70–77 (2008)
Essa M.H.; Al-Zahrani M.A.: Date pits as potential raw materials for the production of active carbons in Saudi Arabia. Int. J. Appl. Environ. Sci. 4, 47–58 (2009)
El-Naas M.H.; Al-Zuhair S.; Alhaija M.A.: Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J. Hazard Mater. 173, 750–757 (2010)
El Nemr A.; Khaled A.; Abdelwahab O.; El-Sikaily A.: Treatment of wastewater containing toxic chromium using new activated carbon developed from date palm seed. J. Hazard Mater. 152, 263–275 (2008)
Girgis B.S.; El-Hendawy A.A.: Porosity development in activated carbons obtained from date pits under chemical activation with phosphoric acid. Micropor. Mesopor. Mater. 52, 105–117 (2002)
Haimour N.M.; Emeish S.: Utilization of date stones for production of activated carbon using phosphoric acid. Waste Manag. 26, 651–660 (2006)
Hameed B.H.; Salman J.M.; Ahmad A.L.: Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J. Hazard Mater. 163, 121–126 (2009)
Jibril B.; Houache O.; Al-Maamari R.; Al-Rashidi B.: Effects of H3PO4 and KOH in carbonization of lignocellulosic material. J. Anal. Appl. Pyrolysis 83, 151–156 (2008)
Merzougui Z.; Addoun F.: Effect of oxidant treatment of date pit activated carbons application to the treatment of waters. Desalination 222, 394–403 (2008)
Riahi K.; Mammou A.B.; Thayer B.B.: Date-palm fibers as a potential technology for tertiary domestic wastewater treatment. J. Hazard Mater. 161, 608–613 (2009)
Riahi K.; Thayer B.B.; Mammou A.B.; Ammar A.B.; Jaafoura M.H.: Biosorption characteristics of phosphates from aqueous solution onto Phoenix dactylifera L. date palm fibers. J. Hazard Mater. 170, 511–519 (2009)
Al-Ghamdi A.; Altaher H.; Omar W.: Application of date palm trunk fibers as adsorbents for removal of Cd +2 ions from aqueous solutions. J. Water Reuse Desalin. 3, 47–54 (2013)
Zhou J.; Yang S.; Yu J.; Shu Z.: Novel hollow microspheres of hierarchical zinc–aluminum layered double hydroxides and their enhanced adsorption capacity for phosphate in water. J. Hazard Mater. 192, 1114–1121 (2011)
Craig A.P.; Franca A.S.; Oliveira L.S.: Evaluation of the potential of FTIR and chemometrics for separation between defective and non-defective coffees. Food Chem. 132, 1368–1374 (2012)
Guo J.; Luo Y.; Lua A.C.; Chi R.; Chen Y.-L.; Bao X.-T.; Xiang S.-X.: Adsorption of hydrogen sulphide (H2S) by activated carbons derived from oil-palm shell. Carbon 45, 330–336 (2007)
Talu O.; Guo C.-J.; Hayhurst D.T.: Heterogeneous adsorption equilibria with comparable molecule and pore sizes. J. Phys. Chem. 93, 7294–7298 (1989)
Das D.; Gaur V.; Verma N.: Removal of volatile organic compound by activated carbon fiber. Carbon 42, 2949–2962 (2004)
El-Hendawy A.A.: Variation in the FTIR spectra of a biomass under impregnation, carbonization and oxidation conditions. J. Anal. Appl. Pyrolysis 75, 159–166 (2006)
Ahmad F.; Daud W.M.A.W.; Ahmad M.A.; Radzi R.; Azmi A.A.: The effects of CO2 activation on porosity and surface functional groups of cocoa (Theobroma cacao) – shell based activated carbon. J. Environ. Chem. Eng. 1, 378–388 (2013)
Nevskaiaa D.M.; Santianesb A.; Munoza V.; Guerrero-Ru’ıza A.: Interaction of aqueous solutions of phenol with commercial activated carbons: an adsorption and kinetic study. Carbon 37, 1065–1074 (1999)
Valdés H.; Solar V.A.; Cabrera E.H.; Veloso A.F.; Zaror C.A.: Control of released volatile organic compounds from industrial facilities using natural and acid-treated mordenites: the role of acidic surface sites on the adsorption mechanism. Chem. Eng. J. 244, 117–127 (2014)
Pan B.; Xing B.: Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 42, 9005–9013 (2008)
Al-Degs Y.; Khraisheh M.A.M.; Allen S.J.; Ahmad M.N.: Effect of carbon surface chemistry on the removal of reactive dyes from textile effluent. Water Res. 34, 927–935 (2000)
Kim K.-J.; Kang C.-S.; You Y.-J.; Chung M.-C.; Woo M.-W.; Jeong W.-J.; Park N.-C.; Ahn H.-G.: Adsorption–desorption characteristics of VOCs over impregnated activated carbons. Catal. Today 111, 223–228 (2006)
Xin Y.; Jinchang L.; Guozhuo G.; Yu J.; Qiang X.: Preparation and modification of activated carbon for benzene adsorption by steam activation in the presence of KOH. Int. J. Min. Sci. Technol. 23, 395–401 (2013)
Abril J.C.; Rodenas M.A.L.; Solano A.L.; Amoros D.C.: Regeneration of activated carbons saturated with benzene or toluene using an oxygen-containing atmosphere. Chem. Eng. Sci. 65, 2190–2198 (2010)
Shim W.G.; Kim S.C.: Heterogeneous adsorption and catalytic oxidation of benzene, toluene, and xylene over spent and chemically regenerated platinum catalyst supported on activated carbon. Appl. Surf. Sci. 256, 5566–5571 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vohra, M.S. Adsorption-Based Removal of Gas-Phase Benzene Using Granular Activated Carbon (GAC) Produced from Date Palm Pits. Arab J Sci Eng 40, 3007–3017 (2015). https://doi.org/10.1007/s13369-015-1683-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-015-1683-0