Abstract
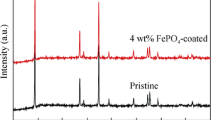
Fe1.5(PO4)(OH) loaded with Au (mass ratio = 0, 0.5, 2, 4, 6 %) was synthesized via two steps, combining microemulsion and hydrothermal techniques, and the effects of the Au loading on the electrochemical properties of Fe1.5(PO4)(OH) cathode material were investigated. The structure and morphology were studied by means of X-ray diffraction (XRD) and field emission-scanning electron microscopy (FE-SEM), and the electrochemical performances were characterized by galvanostatic charge and discharge tests. The XRD patterns showed that the Au loading modification did not affect the structure of Fe1.5(PO4)(OH). From the charge and discharge test, it was found that when the mass ratio of Au loading was 2 % the sample exhibited excellent electrochemical properties with an initial specific capacity of 182 mAhg−1 at 0.1 C and less fading of the specific capacity, retaining about 165 mAhg−1 after 25 cycles, which demonstrated that the Au loading is an effective way to improve the electron conductivity of Fe1.5(PO4)(OH). What is more, the electrochemical properties of Fe1.5(PO4)(OH)/Au synthesized at 150 °C for 24 h with a discharge specific capacity of 182 mAhg−1 are better than those of the sample synthesized at 180 °C for 24 h with a discharge specific capacity of 121 mAhg−1, which is attributed to the crystal growth of particles at high hydrothermal temperatures, showing that the particle size also has a significant influence on the electrochemical performance of electrode materials.

Similar content being viewed by others
References
Wang, Y.; Cao, G.: Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv. Mater. 20, 2251–2269 (2008)
Grigoryev, E.: Kinetics of densification processes of powder materials under electropulse sintering. Arab. J. Sci. Eng. 34, 29–33 (2009)
Padhi, A.; Nanjundaswamy, K.;Goodenough, J.B.: Phospho- as positive-materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188–1194 (1997)
Allen, J.; Jow, T.;Wolfenstine, J.: Analysis of the FePO4 to LiFePO4 phase transition. J. Solid State Electrochem. 12, 1031–1033 (2008)
Song, Y.; Zavalij, P.Y.; Suzuki, M.; Whittingham, M.S.: New iron (III) phosphate phases: crystal structure and electrochemical and magnetic properties. Inorg. chem. 41, 5778–5786 (2002)
Gerbaldi, C.; Meligrana, G.; Bodoardo, S.; Tuel, A.; Penazzi, N.: FePO4 nanoparticles supported on mesoporous SBA-15: interesting cathode materials for Li-ion cells. J. Power Sources 174, 501–507 (2007)
Ryu, J.; Park, C.B.; Kang, K.: Carbon nanotube-amorphous FePO4 core–shell nanowires as cathode material for Li ion batteries. Chem. Commun. 46, 7409–7411 (2010)
Yang, X.; Zhang, S.; Zhang, J.; Xu, M.; Ren, P.; Li, X.; Yan, L.: The study on synthesis and modification for iron phosphate. Funct. Mater. Lett. 4, 323–326 (2011)
Lindberg, M.; Christ, C.: Crystal structures of the isostructural minerals lazulite, scorzalite and barbosalite. Acta Crystallographica 12, 695–697 (1959)
Redhammer, G.; Tippelt, G.; Roth, G.; Lottermoser, W.; Amthauer, G.: Structure and mössbauer spectroscopy of barbosalite Fe2+ Fe3+ 2(PO4)2(OH)2 between 80K and 300K. Phys. Chem. Miner. 27, 419–429 (2000)
Moore, P.B.: Basic ferric phosphates: a crystallochemical principle. Sci. (New York, NY) 164, 1063–1064 (1969)
Fanfani, I.; Zanazzi, P.: The crystal structure of beraunite. Acta Crystallographica 22, 173–181 (1967)
Moore, P.B.; Kampf, A.R.: Beraunite: refinement, comparative crystal chemistry, and selected bond valences. Zeitschrift für Kristallographie 201, 263–281 (1992)
Song, Y.; Zavalij, P.Y.; Chernova, N.A.; Whittingham, M.S.: Synthesis, crystal structure, and electrochemical and magnetic study of new iron (III) hydroxyl-phosphates, isostructural with lipscombite. Chem. Mater. 17, 1139–1147 (2005)
Yang, H.; Costin, G.; Keogh, J.; Lu, R.; Downs, R.T.: Cobaltaustinite, CaCo(AsO4)(OH), Acta Crystallographica Section E: structure reports online, 63, i53-i55 (2007)
Dollé, M.; Patoux, S.; Richardson, T.J.: Lithium insertion chemistry of phosphate phases with the lipscombite structure. J. Power Sources 144, 208–213 (2005)
Wang, Z.; Sun, S.; Li, F.; Chen, G.; Xia, D.; Zhao, T.; Chu, W.; Wu, Z.: Stability, electrochemical behaviors and electronic structures of iron hydroxyl-phosphate. Mater. Chem. Phys. 123, 28–34 (2010)
Moon, S.J.; Kouh, T.; Lee, C.S.; Kim, C.S.: Investigation of microscopic crystal field in Co-doped lithium-iron phosphate. IEEE Trans. Magn. 45, 2584–2586 (2009)
Myung, S.T.; Amine, K.; Sun, Y.K.: Surface modification of cathode materials from nano-to microscale for rechargeable lithium-ion batteries. J. Mater. Chem. 20, 7074–7095 (2010)
Hu, J.; Xie, J.; Zhao, X.; Yu, H.; Zhou, X.; Cao, G.; Tu, J.: Doping effects on electronic conductivity and electrochemical performance of LiFePO4. J. Mater. Sci. Technol. 25, 405–409 (2009)
Mi, C.H.; Cao, Y.X.; Zhang, X.G.; Zhao, X.B.; Li, H.L.: Synthesis and characterization of LiFePO4/(Ag+C) composite cathodes with nano-carbon webs. Powder Technol. 181, 301–306 (2008)
Chang, H.H.; Chang, C.C.; Su, C.Y.; Wu, H.C.; Yang, M.H.; Wu, N.L.: Effects of TiO2 coating on high-temperature cycle performance of LiFePO4-based lithium-ion batteries. J. Power Sources 185, 466–472 (2008)
Zou, B.; Wang, Y.; Zhou, S.: Spray drying-assisted synthesis of LiFePO4/C composite microspheres with high performance for lithium-ion batteries. Mater. Lett. 92, 300–303 (2013)
Malik, R.; Burch, D.; Bazant, M.; Ceder, G.: Particle size dependence of the ionic diffusivity. Nano Lett. 10, 4123–4127 (2010)
Padhi, A.; Nanjundaswamy, K.; Masquelier, C.; Okada, S.; Goodenough, J.: Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates. J. Electrochem. Soc. 144, 1609–1613 (1997)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S.M., Zhang, J.X., Xu, S.J. et al. The Effects of Au Loading on the Electrochemical Properties of Fe1.5(PO4)(OH) Cathode Material for Lithium-Ion Batteries. Arab J Sci Eng 39, 6643–6649 (2014). https://doi.org/10.1007/s13369-014-1181-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-014-1181-9