Abstract
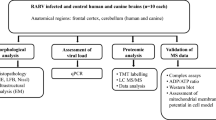
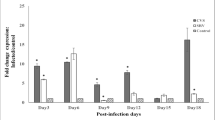
Our previous work in a mouse model of experimental rabies showed neuronal process (dendrites and axons) degeneration in association with severe clinical disease. Cultured adult rodent dorsal root ganglion (DRG) neurons infected with the challenge virus standard-11 (CVS) strain of rabies virus (RABV) showed axonal swellings and reduced axonal growth with evidence of oxidative stress. We have shown that CVS infection alters a variety of mitochondrial parameters and increases mitochondrial complex I activity and reactive oxygen species (ROS) production. Expression of a peptide from amino acid 139–172 of the CVS phosphoprotein (P) increased complex I activity and ROS generation similar to expression of the entire P. Site-directed mutational analyses illustrated the importance of the 145–151 and 157–169 regions of P and that serine residues at 162 and 166 are important single amino acid sites. Two CVS recombinant viruses with serine to alanine mutations at positions 162 (A162r) and 166 (A166r) did not increase complex I activity or ROS generation and also did not induce axonal swellings or inhibit axonal growth in DRG neurons. RABV infection is a mitochondrial disorder initiated by interaction of the RABV P and complex I; S162 and S166 are critical sites in the P for this interaction. The resulting mitochondrial dysfunction produces oxidative stress in neurons causing acute degenerative changes affecting neuronal processes resulting in a severe and fatal clinical disease. This information will be important for the future development of novel therapies for rabies.







Similar content being viewed by others
References
Alandijany T, Kammouni W, Roy Chowdhury SK, Fernyhough P, Jackson AC (2013) Mitochondrial dysfunction in rabies virus infection of neurons. J Neurovirol 19:537–549
Dawson TM, Ko HS, Dawson VL (2010) Genetic animal models of Parkinson’s disease. Neuron 66:646–661
De BP, Banerjee AK (1997) Role of host proteins in gene expression of nonsegmented negative strand RNA viruses. Adv Virus Res 48:169–204
De BP, Das T, Banerjee AK (1997) Role of cellular kinases in the gene expression of nonsegmented negative strand RNA viruses. Biol Chem 378:489–493
Fu ZF, Jackson AC (2005) Neuronal dysfunction and death in rabies virus infection. J Neurovirol 11:101–106
Gholami A, Kassis R, Real E, Delmas O, Guadagnini S, Larrous F, Obach D, Prevost MC, Jacob Y, Bourhy H (2008) Mitochondrial dysfunction in lyssavirus-induced apoptosis. J Virol 82:4774–4784
Gupta AK, Blondel D, Choudhary S, Banerjee AK (2000) The phosphoprotein of rabies virus is phosphorylated by a unique cellular protein kinase and specific isomers of protein kinase C. J Virol 74:91–98
Huang G, Lu H, Hao A, Ng DC, Ponniah S, Guo K, Lufei C, Zeng Q, Cao X (2004) GRIM-19, a cell death regulatory protein, is essential for assembly and function of mitochondrial complex I. Mol Cell Biol 24:8447–8456
Huntley CC, De BP, Murray NR, Fields AP, Banerjee AK (1995) Human parainfluenza virus type 3 phosphoprotein: identification of serine 333 as the major site for PKC zeta phosphorylation. Virology 211:561–567
Iwasaki Y, Tobita M (2002) Pathology. In: Jackson AC, Wunner WH (eds) Rabies. Academic Press, San Diego, pp. 283–306
Jackson AC (2013a) Current and future approaches to the therapy of human rabies. Antivir Res 99:61–67
Jackson AC (ed) (2013b) Rabies: scientific basis of the disease and its management, Third edn. Elsevier Academic, Oxford
Jackson AC (2013c) Therapy of human rabies. In: Jackson AC (ed) Rabies: scientific basis of the disease and its management, Third edn. Elsevier Academic, Oxford, pp. 573–587
Jackson AC, Kammouni W, Zherebitskaya E, Fernyhough P (2010) Role of oxidative stress in rabies virus infection of adult mouse dorsal root ganglion neurons. J Virol 84:4697–4705
Jackson AC, Reimer DL, Ludwin SK (1989) Spontaneous recovery from the encephalomyelitis in mice caused by street rabies virus. Neuropathol Appl Neurobiol 15:459–475
Kammouni W, Hasan L, Saleh A, Wood H, Fernyhough P, Jackson AC (2012). Role of nuclear factor-kB in oxidative stress associated with rabies virus infection of adult rat dorsal root ganglion neurons. J Virol 86:8139-8146.
Kammouni W, Wood H, Saleh A, Appolinario CM, Fernyhough P, Jackson AC (2015) Rabies virus phosphoprotein interacts with mitochondrial complex I and induces mitochondrial dysfunction and oxidative stress. J Neurovirol 21:370–382
Kuan WL, Poole E, Fletcher M, Karniely S, Tyers P, Wills M, Barker RA, Sinclair JH (2012) A novel neuroprotective therapy for Parkinson’s disease using a viral noncoding RNA that protects mitochondrial complex I activity. J Exp Med 209:1–10
Lieu KG, Brice A, Wiltzer L, Hirst B, Jans DA, Blondel D, Moseley GW (2013). The rabies virus interferon antagonist P protein interacts with activated STAT3 and inhibits Gp130 receptor signaling. J Virol 87:8261-8265.
Lu H, Cao X (2008) GRIM-19 is essential for maintenance of mitochondrial membrane potential. Mol Biol Cell 19:1893–1902
Nishi H, Hashimoto K, Panchenko AR (2011) Phosphorylation in protein-protein binding: effect on stability and function. Structure 19:1807–1815
Pattnaik AK, Hwang L, Li T, Englund N, Mathur M, Das T, Banerjee AK (1997) Phosphorylation within the amino-terminal acidic domain I of the phosphoprotein of vesicular stomatitis virus is required for transcription but not for replication. J Virol 71:8167–8175
Raux H, Flamand A, Blondel D (2000) Interaction of the rabies virus P protein with the LC8 dynein light chain. J Virol 74:10212–10216
Reeves MB, Davies AA, McSharry BP, Wilkinson GW, Sinclair JH (2007) Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 316:1345–1348
Rossiter JP, Jackson AC (2013) Pathology. In: Jackson AC (ed) Rabies: scientific basis of the disease and its management, Third edn. Elsevier Academic, Oxford, pp. 351–386
Scott CA, Rossiter JP, Andrew RD, Jackson AC (2008) Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome in experimental rabies in yellow fluorescent protein-expressing transgenic mice. J Virol 82:513–521
Szczepanek K, Chen Q, Larner AC, Lesnefsky EJ (2012) Cytoprotection by the modulation of mitochondrial electron transport chain: the emerging role of mitochondrial STAT3. Mitochondrion 12:180–189
Xue X, Zheng X, Liang H, Feng N, Zhao Y, Gao Y, Wang H, Yang S, Xia X (2014) Generation of recombinant rabies virus CVS-11 expressing eGFP applied to the rapid virus neutralization test. Viruses 6:1578–1589
Zhang G, Wang H, Mahmood F, Fu ZF (2013) Rabies virus glycoprotein is an important determinant for the induction of innate immune responses and the pathogenic mechanisms. Vet Microbiol 162:601–613
Zherebitskaya E, Akude E, Smith DR, Fernyhough P (2009). Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes 58:1356-1364.
Acknowledgements
We wish to acknowledge Michael Carpenter for assistance with the western blots, Stephanie Booth and Anna Majer for assistance with confocal microscopy, and Kym Antonation and Chantel Urfano for sequencing the RABV virus genomes (all at the Public Health Agency of Canada, Winnipeg, Manitoba, Canada).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding statement
This work was supported by a Research Manitoba Bridge Funding Award with the Department of Internal Medicine, University of Manitoba (to A.C. Jackson).
Conflict of interest
Wafa Kammouni, Heidi Wood, and Alan Jackson declare they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kammouni, W., Wood, H. & Jackson, A.C. Serine residues at positions 162 and 166 of the rabies virus phosphoprotein are critical for the induction of oxidative stress in rabies virus infection. J. Neurovirol. 23, 358–368 (2017). https://doi.org/10.1007/s13365-016-0506-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-016-0506-8