Abstract
Eurasian lynx is one of most widely distributed felid species. However, populations in central Europe are strongly fragmented and limited to forest areas, which may influence their sustainability and gene flow. We studied the edge effect on mortality of a highly isolated population of Eurasian lynx in the Białowieża Primeval Forest (NE Poland) during the 20-year period (1991–2011) of high economic development in Poland. Based on radio-tracking data collected in 1991–1996 (low gross national income per capita; low GNI PC) and 2004–2011 (high GNI PC) and recorded mortality cases, we analysed annual rates and causes of mortality and spatial distribution of death sites relative to the distance from the forest edge. We found significantly higher mortality of lynx during the low GNI PC phase of the economic growth than during high GNI PC (33 and 16 %, respectively). While anthropogenic factors played a dominant role in lynx mortality during low GNI PC, natural factors prevailed afterwards. We found a significantly higher proportion of lynx deaths than expected in the edge and outer zone than in the core of the Białowieża Forest. Both areas differ significantly in the proportions of mortality causes with anthropogenic factors being the main source of mortality in the outer zone. Our results indicate that the decline of lynx mortality in Poland could be related to the improving of the economic situation in the country after the collapse of communism and reduced significance of anthropogenic factors (mainly poaching). Additionally, the outskirts of the forest may function as population sinks, which may reduce the dispersal of lynx and gene flow between populations inhabiting different forest patches.
Similar content being viewed by others
Introduction
Large carnivores live in low densities and roam over long distances; therefore, vast areas are essential for long-term viability of their populations. Due to fragmentation and the limited range of suitable environments, they are vulnerable to wildlife-human conflicts and human plays a major role in their mortality (Woodroffe and Ginsberg 1998; Andrén et al. 2006; Mondal et al. 2013). The risk of mortality increases when carnivores roam beyond protected areas or on the edges of their distribution range (Woodroffe and Ginsberg 1998). Therefore, such outer areas may act as population sinks (Delibes et al. 2001; Bunnefeld et al. 2006).
The Eurasian lynx (Lynx lynx) is one of most widely distributed felid species. However, in central Europe, their populations are strongly fragmented and limited to forest areas (Schmidt 1998; Von Arx et al. 2004; Niedziałkowska et al. 2006). The ranging and dispersal routes of lynx have been shown to be dependent on the distribution and availability of woodlands (Schmidt et al. 1997; Schmidt 1998; Podgórski et al. 2008). Recent studies on lynx genetic diversity and differentiation have suggested that closely situated but isolated populations are poorly connected through contemporary gene flow (Schmidt et al. 2009, 2011; Ratkiewicz et al. 2012), indicating the possible effect of habitat fragmentation on the effectiveness of lynx dispersal. Studies in Scandinavia have shown that human-induced factors (harvest, poaching, vehicle collisions) are the chief mortality causes in this species (Andrén et al. 2006) and that human-dominated areas represent a potentially high mortality risk for the lynx (Bunnefeld et al. 2006). These factors could explain the causal link between the habitat fragmentation and the lynx genetic structure; however, the situation of this species in central Europe is different due to its legal protection status (Von Arx et al. 2004). Interestingly, the lynx populations in Scandinavia and Baltic states are increasing and expanding (Linnell et al. 2005, 2010) despite being subject to hunting harvest, whereas those in central Europe (Poland, Lithuania and Belarus) stay constant or even decline under legal protection (Linnell et al. 2005). Therefore, it seems that legal protection is not a sufficient conservation measure to warrant the increase of effective dispersal in these populations (Niedziałkowska et al. 2006).
The population status of the lynx within Poland requires special attention because its range has decreased during the period between 1980 and 2001 (Jędrzejewski et al. 2002) and has not recovered until present despite being an officially protected species in Poland since 1995 (unpublished data of the Mammal Research Institute collected under the National Survey of Large Carnivores in Poland). Although this may be partly due to habitat fragmentation (Schmidt et al. 2011), the mechanism behind it is unclear. As the lynx in Poland reaches the westernmost edge of the species natural range (i.e. excluding the reintroduced populations) in Europe, this area is crucial for the conservation and potential expansion of this predator westwards. Unravelling the factors limiting lynx dispersal in this range may help to preserve the genetic diversity and effective gene flow between the populations (Garner et al. 2005; Ratkiewicz et al. 2012).
In this study, we aimed at evaluating the effects of two factors potentially influential to the mortality in the lynx population in the Białowieża Primeval Forest (NE Poland): location relative to the forest edge (as a proxy of the human-related mortality risk) and the economic situation of the country (as a proxy of the poaching pressure).
Material and methods
Study area
The study was conducted in the Białowieża Primeval Forest (=BPF, 52° 29′–52° N, 23° 31′–24° 21′ E, northeastern Poland)—one of the best preserved lowland forests in Europe, located on the Polish-Belarusian border. It covers 1500 km2. In the Polish part of the forest (600 km2), deciduous and mixed tree stands (mainly pine Pinus sylvestris, spruce Picea abies, alder Alnus glutinosa and oak Quercus robur) cover 94 % of the BPF, while open habitats constitute 6 % of the area (Sokołowski 2004). From the west and north, the BPF is surrounded by open habitats dominated by pastures and meadows (41 % of open area) and arable lands (59 %) interspersed with settlements and small woodlands (Hofman-Kamińska and Kowalczyk 2012). The region is characterised by extensive agriculture and low human density (30 people/km2; Demographic Yearbook of Poland 2009). The majority of human settlements are localised on the edges and outside of the forest, with very few villages being localised within the forest. The climate of the Białowieża Forest is transitional between Atlantic and Continental types. The mean annual temperature is 7 °C, with July being the warmest month (average 18.4 °C) and January the coldest (average −4.8 °C) (Jędrzejewska and Jędrzejewski 1998). Snow cover persists from 60 to 96 days in the BPF with the max recorded depth of 95 cm.
Data collection and analysis
We investigated all cases of lynx mortalities that were found in the Białowieża Primeval Forest and its vicinities during the telemetry study (using both VHF and GPS transmitters) conducted in 1991–2011 (Schmidt et al. 1997; Schmidt 2008; Davoli et al. 2013; Schmidt K. and Kowalczyk R. unpublished results). The telemetry data were used to calculate the survival and mortality rates, whereas the data on casualties of both collared and uncollared lynx were used for analyses of mortality sources and spatial distribution. Lynx were intensively monitored by telemetry in two periods: 1991–1996 and 2004–2011. The two study periods referred to different phases of economic development of the country with the former corresponding to low average gross national income per capita (GNI PC) = US$6557 and the latter to high average GNI PC = US$16,534 (World Bank data—data.worldbank.org/country/poland). Lynx was not a protected species before 1995 in Poland, though it was locally protected in the BPF since 1989.
In total, 33 lynx were monitored with telemetry (18 in 1991–1996 and 15 in 2004–2011) for an average period of 394 ± 219 days (range 30–855). The duration of tracking did not differ between the two periods (Mann-Whitney test, P > 0.1). Thirteen collared individuals were found dead, nine during the period for which the mortality and survival rates were calculated and four out of this period. Additional data on lynx mortality included 13 uncollared dead lynx that were found occasionally. Most of the casualties were inspected by veterinarians to determine the source of mortality. We described mortality sources as natural (disease, starvation, predation, etc.) or anthropogenic (poaching, car accidents, etc.). All mortalities were classified as being caused by natural factors if there were no unambiguous signs of human influence involved. Cause-specific mortality and survival rates were estimated with MICROMORT software (Heisey and Fuller 1985) on the basis of radio-days for 33 lynx and recorded mortalities of 9 collared lynx.
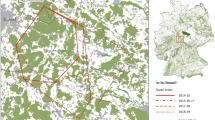
Locations of all recorded lynx mortalities between 1991 and 2011 were analysed in relation to their distribution in the core and outer area of the BPF. We included in the outer area a 2-km buffer zone of the forest peripheries to account for potential influence of human activity penetrating into the forest (Fig. 1). To estimate the expected distribution of lynx mortalities in the core and outer area, we calculated the proportion of collared lynx localisations in both areas. To make results of VHF and GPS tracking comparable, we randomly reduced (using Excel spreadsheet) the number of localisations for lynx tracked with GPS collars to obtain the same number of radio-fixes per day as for lynx tracked using VHF collars (0.65 localization/day). In total, 11,043 localisations (8673 in the core and 2370 in the outer area) of collared lynx were used in the analysis.
Spatial distribution of collared lynx localisations and recorded mortalities (including collared and uncollared lynx) in the Białowieża Primeval Forest in 1991–2011 in relation to the core and outer area of the forest. Core area is determined by excluding a 2-km buffer zone of the forest peripheries to account for potential influence of human activity penetrating into the forest. White background is the area used for agriculture and human settlements. Symbol in the left upper corner of the figure denotes location of collared lynx found dead 25 km away from the area showed on the map
Results
Human-induced mortality accounted for 57.7 % of total mortality cases (n = 26) recorded during the study period in the BPF and its vicinities. Poaching constituted 66.7 % of all human-related mortalities.
Annual lynx mortality estimated on the basis of radio-tracking data was higher during the low GNI PC period (33 %) (1991–1996) than that during the high GNI PC (16 %) (2004–2011) (G-test; G = 6.02, df = 1, p < 0.02) (Table 1). There was a reversed pattern of cause-specific mortality rates recorded in the two periods, with anthropogenic factors playing a dominating role during the first period and natural factors during the second phase of economic growth (G-test; G = 13.08, df = 1, p < 0.001) (Table 1). A similar pattern was observed when all lynx deaths (collared and uncollared) were jointly analysed. The significance of anthropogenic mortality declined from 66.7 % during the period of low GNI PC to 45.5 % during high GNI PC (G-test, G = 9.20, df = 1, p < 0.01) (Fig. 2).
We found a strong edge effect on mortality of lynx. While in the outer area of the forest the proportion of lynx deaths was significantly higher than expected (65.4 versus 21.5 %), within the core area it was significantly lower than expected (34.6 vs. 78.5 %) (G-test, G = 40.73, df = 1, p < 0.001) (Fig. 3). The lynx deaths in the outer zone were nearly twice as high as those in the core area (9 versus 17 cases of deaths recorded, respectively), and both areas significantly differed in proportion of anthropogenic and natural sources of mortality (G-test, G = 8.29, df = 1, p < 0.01), with human-induced mortality playing a significantly more important role in the outer (64.7 % of death cases) than that in the core area (44.5 %) (Figs. 1 and 4).
Expected (grey bars; based on proportion of radio and GPS localisations of collared lynx in the core and outer area; see Fig. 1) and observed (black bars) distribution of lynx mortalities in the core and outer areas of the Białowieża Primeval Forest in 1991–2011
Discussion
Our study showed how the combination of two factors, human-induced mortality and habitat fragmentation, may have influenced the population of lynx being under official protection. Although the species is protected in Poland since 1995 and locally in the Białowieża Forest since 1989, human-related factors, mainly poaching, were the most important source of lynx mortality in the early 1990s (Jędrzejewski et al. 1996). Our study period has spanned 20 years beginning shortly after the collapse of communism in Poland and is long enough to observe the effects of increasing economic prosperity in the country on the effectiveness of conservation of this large felid.
The period before political and economic changes in Poland was characterised with substantial poverty, particularly in rural areas (Kostova-Huffman and Johnson 2004). This was likely a causal factor behind poaching, as it provided additional food resources and income for peasants. Although poachers mainly targeted ungulates, the animals were usually killed with snares, in which the lynx occasionally became trapped (Jędrzejewski et al. 1996; Schmidt et al. 1997). Poaching of ungulates could have had a substantial detrimental effect on all wildlife, as it was widespread in forested areas in Poland and other post-Soviet countries during communist times and also during the first decade after the democratic transformation in early 1990s, mainly due to food rationing in 1970–80s (Jędrzejewski et al. 2005). We presume that the more than twofold increase in the income per capita, which occurred during the second period of our study, could have caused a decline in the need for additional resources by rural societies and indirectly to the decline of lynx mortality in Poland. The shift from mainly anthropogenic-caused mortality to mainly natural-caused mortality between these two economic phases may farther contribute to improving the conservation status of these felids in Poland.
Interestingly, in Scandinavia and Switzerland, anthropogenic causes of mortality, including hunting and poaching, were also much greater than natural sources of mortality (Schmidt-Posthaus et al. 2002; Andrén et al. 2006). Also, in other species of carnivores, such as wolves and wolverines in Scandinavia or tigers in southern Asia, human-related factors, mainly poaching, played the most significant role accounting for 51–83 % of mortality cases (Liberg et al. 2012; Goodrich et al. 2008; Persson et al. 2009). This increased mortality due to poaching and other human-related factors may significantly influence the survival of large carnivores and drive local populations to extinction (Chapron et al. 2008). However, despite high levels of anthropogenic-caused mortality, the Scandinavian lynx population has showed a tendency to increase and expand (Linnell et al. 2010; Nilsen et al. 2012). Therefore, as there is no extra lynx mortality due to hunting harvest in Poland, we presume that there must be other factors responsible for the lack of population increase under legal protection.
The risk of mortality increases when carnivores roam beyond protected areas or on the edges of their distribution range (Woodroffe and Ginsberg 1998). Our study provided a clear support for the edge effect in patterns of lynx mortality, with anthropogenic factors playing a dominant role in outer areas. Lynx was indeed found to show an apparent perception of the risk related with open agricultural areas in the hunted population in Norway (Bunnefeld et al. 2006; Basille et al. 2009). These areas were considered to represent the “attractive sinks” due to high abundance of the main lynx prey—the roe deer (Okarma et al. 1997)—on the one hand and high mortality there due to hunting harvest on the other. In our study area, roe deers are also abundant on the outskirts of the Białowieża Forest (Sönnichsen et al. 2013), and therefore, these areas may be potentially important for lynx. Although no legal harvest for lynx occurs in the BPF, the human-dominated area still seems to act as a population sink, although this is mostly due to poaching. Such population sinks may effectively reduce the dispersal rate of lynx and thus the gene flow between neighbouring populations inhabiting different forest patches. Indeed, based on mtDNA haplotype frequencies, it was found that the lynx population from the BPF was significantly divergent from neighbouring populations (Ratkiewicz et al. 2012). Furthermore, it was characterised by a relatively low allelic richness (Schmidt et al. 2009) when compared to Latvian and Estonian lynx which populate more continuous habitats. Our study suggests that the effect of habitat fragmentation on the lynx population is probably reinforced by higher human-induced mortality in areas between forest patches occupied by lynx. Edge effect related to accidental and deliberate human-induced mortality of carnivores when ranging beyond the protected or core distribution areas was reported also for other species of carnivores such as leopards, lions or badgers (Revilla et al. 2001; Loveridge et al. 2007; Balme et al. 2010).
In contrast to the common assumption about the inverse relationship between the economic development of the country and the effectiveness of nature protection (Tisdell 1999), our report presents a case of a possible positive effect of economic growth in decreasing the mortality of a large predator. However, fragmentation of lynx habitat may still be the main factor hampering the population increase and expansion due to strong edge effects in their mortality on limiting dispersal and gene flow amongst populations. Therefore, this result emphasises the necessity of restoring habitat connectivity by ecological corridors as one of the most important conservation measures for successful protection of this felid.
References
Andrén H, Linnell JDC, Liberg O, Andersen R, Danell A, Karlsson J, Odden J, Moa PF, Ahlqvist P, Kvam Y, Franzén F, Segerström P (2006) Survival rates and causes of mortality in Eurasian lynx (Lynx lynx) in multi-use landscapes. Biol Conserv 131:23–32
Balme GA, Slotow R, Hunter LTB (2010) Edge effects and the impact of non-protected areas in carnivore conservation: leopards in the Phinda–Mkhuze Complex, South Africa. Anim Conserv 13:315–323
Basille M, Herfindal I, Santin-Janin H, Linnell JDC, Odden J, Andersen R, Høgda KA, Gaillard J-M (2009) What shapes Eurasian lynx distribution in human dominated landscapes: selecting prey or avoiding people? Ecography 32:683–691
Bunnefeld N, Linnell JDC, Odden J, Van Duijn MAJ, Andersen R (2006) Risk taking by Eurasian lynx (Lynx lynx) in a human-dominated landscape: effects of sex and reproductive status. J Zool 270:31–39
Chapron G, Miquelle DG, Lambert A, Goodrich JM, Legendre S, Clobert J (2008) The impact on tigers of poaching versus prey depletion. J Appl Ecol 45:1667–1674
Davoli F, Schmidt K, Kowalczyk R, Randi E (2013) Hair snaring and molecular genetic identification for reconstructing the spatial structure of Eurasian lynx populations. Mamm Biol 78:118–126
Delibes M, Goana P, Ferreras P (2001) Effects of an attractive sink leading into maladaptive habitat selection. Am Nat 158:277–285
Garner A, Rachlow JL, Hicks JF (2005) Patterns of genetic diversity and its loss in mammalian populations. Conserv Biol 19:1215–1221
Goodrich JM, Kerley LL, Smirnov EN, Miquelle DG, McDonald L, Quigley HB, Hornocker MG, McDonald T (2008) Survival rates and causes of mortality of Amur tigers on and near the Sikhote-Alin Biosphere Zapovednik. J Zool 276:323–329
Heisey DM, Fuller TK (1985) Evaluation of survival and cause-specific mortality rates using telemetry data. J Wildl Manage 49:668–674
Hofman-Kamińska E, Kowalczyk R (2012) Farm crops depredation by European bison (Bison bonasus) in the vicinity of forest habitats in northeastern Poland. Environ Manage 50:530–541
Jędrzejewska B, Jędrzejewski W (1998) Predation in vertebrate communities. The Białowieża Primeval Forest as a case study. Springer, Berlin
Jędrzejewski W, Jędrzejewska B, Okarma H, Schmidt K, Bunevich AN, Miłkowski L (1996) Population dynamics (1869–1994), demography and home ranges of the lynx in Białowieża Primeval Forest (Poland and Belarus). Ecography 19:122–138
Jędrzejewski W, Branicki W, Veit C, Medugorac I, Pilot M, Bunevich AN, Jędrzejewska B, Schmidt K, Theuerkauf J, Okarma H, Gula R, Szymura L, Förster M (2005) Genetic diversity and relatedness within packs in an intensely hunted population of wolves Canis lupus. Acta Theriol 50:1–22
Jędrzejewski W, Nowak S, Schmidt K, Jędrzejewska B (2002) The wolf and the lynx in Poland—results of a census conducted in 2001. Kosmos 51:491–499
Kostova-Huffman S, Johnson SR (2004) Impacts of economic reform in Poland: incidence and welfare changes within a consistent framework. Rev Econ Stat 86:626–636
Liberg O, Chapron G, Wabakken P, Pedersen HC, Hobbs NT, Sand H (2012) Shoot, shovel and shut up: cryptic poaching slows restoration of a large carnivore in Europe. Proc R Soc B 279:910–915
Linnell JDC et al. (2005) Large carnivores in northern landscapes: final report. Status survey, conflicts, human dimensions, ecology and conservation of bears, lynx and wolves in Estonia, Latvia, Lithuania and Poland. Final report. Norwegian Institute for Nature Research, Trondheim
Linnell JDC, Broseth H, Odden J, Nilsen E (2010) Sustainably harvesting a large carnivore? Development of Eurasian lynx populations in Norway during 160 years of shifting policy. Environ Manage 45:1142–1154
Loveridge AJ, Searle AW, Murindagomo F, MacDonald DW (2007) The impact of sport-hunting on the population dynamics of an African lion population in a protected area. Biol Conserv 134:548–558
Mondal K, Sankar K, Qureshi Q (2013) Factors influencing the distribution of leopard in a semiarid landscape of Western India. Acta Theriol 58:179–187
National Survey of Large Carnivores in Poland 2000–2012 - http://www.zbs.bialowieza.pl/artykul/528.html
Niedziałkowska M, Jędrzejewski W, Mysłajek RW, Nowak S, Jędrzejewska B, Schmidt K (2006) Environmental correlates of Eurasian lynx occurrence in Poland—large scale census and GIS mapping. Biol Conserv 133:63–69
Nilsen EB, Brøseth H, Odden J, Linnell JDC (2012) Quota hunting of Eurasian lynx in Norway: patterns of hunter selection, hunter efficiency and monitoring accuracy. Eur J Wildl Res 58:325–333
Okarma H, Jędrzejewski W, Schmidt K, Kowalczyk R, Jędrzejewska B (1997) Predation of Eurasian lynx on roe deer and red deer in Białowieża Primeval Forest, Poland. Acta Theriol 42:203–224
Persson J, Ericsson G, Segerström P (2009) Human caused mortality in the endangered Scandinavian wolverine population. Biol Conserv 142:325–331
Podgórski T, Schmidt K, Kowalczyk R, Gulczyńska A (2008) Microhabitat selection by Eurasian lynx and its implications for species conservation. Acta Theriol 53:97–110
Ratkiewicz M, Matosiuk M, Kowalczyk R, Konopiński MK, Okarma H, Ozolins J, Männil P, Ornicans A, Schmidt K (2012) High levels of population differentiation in Eurasian lynx at the edge of the species’ western range in Europe revealed by mitochondrial DNA analyses. Anim Conserv 15:603–612
Revilla E, Palomares F, Delibes M (2001) Edge-core effects and the effectiveness of traditional reserves in conservation: Eurasian badgers in Doñana National Park. Conserv Biol 15:148–158
Schmidt K (1998) Maternal behaviour and juvenile dispersal in the Eurasian lynx. Acta Theriologica 43:391–401
Schmidt K (2008) Behavioural and spatial adaptation of the Eurasian lynx to a decline in prey availability. Acta Theriol 53:1–16
Schmidt K, Jędrzejewski W, Okarma H (1997) Spatial organization and social relations in the Eurasian lynx population in Białowieża Primeval Forest, Poland. Acta Theriol 42:289–312
Schmidt K, Kowalczyk R, Ozolins J, Männil P, Fickel J (2009) Genetic structure of the Eurasian lynx population in north-eastern Poland and the Baltic states. Conserv Gen 10:497–501
Schmidt K, Ratkiewicz M, Konopiński MK (2011) The importance of genetic variability and population differentiation in the Eurasian lynx Lynx lynx for conservation, in the context of habitat and climate change. Mamm Rev 41:112–124
Schmidt-Posthaus H, Breitenmoser-Würsten C, Posthaus H, Bacciarini L, Breitenmoser U (2002) Causes of mortality in reintroduced Eurasian lynx in Switzerland. J Wildl Dis 38:84–92
Sokołowski AW (2004) Woods of the Białowieża Forest. State Forests Information Centre, Warszawa
Sönnichsen L, Bokje M, Marchal J, Hofer H, Jędrzejewska B, Kramer-Schadt S, Ortmann S (2013) Behavioural responses of European roe deer to temporal variation in predation risk. Ethology 119:233–243
Tisdell C (1999) Biodiversity, conservation and sustainable development, principles and practices with Asian examples. Edward Elgar Publishing Ltd, Cheltenham
Von Arx M, Breitenmoser-Würsten C, Zimmermann F, Breitenmoser U (2004) Status and conservation of the Eurasian lynx (Lynx lynx) in 2001. KORA Bericht 19:1–319
Woodroffe R, Ginsberg JR (1998) Edge effects and the extinction of populations inside protected areas. Science 280:2126–2128
World Bank data—data.worldbank.org/country/poland
Acknowledgments
The study was financed by the Polish Ministry of Sciences grant nos. 6P205 034 05 and 3P04F 019 24 and the statutory budget of the Mammal Research Institute PAS. This work was partly based on published data collected in collaboration with dr W. Jędrzejewski and H. Okarma. Special thanks go to E. Bujko for his help in trapping and radio-tracking lynx. The following students and volunteers also assisted in the study: T. Podgórski, A. Gulczyńska, L. Sönnichsen, E. Schutz, M. Adams, M. Kruit, C. Conti, A.M. Nuno, A. Nees, B. Cristescu and P. Vallo. Permission for lynx trapping was issued by the Ministry of Environment. Trapping, handling and radio-tracking procedures were approved by the Polish Ethical Commission for Research on Animals. We are grateful to Ms. Elizabeth Finch for correcting the English of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Karol Zub
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kowalczyk, R., Górny, M. & Schmidt, K. Edge effect and influence of economic growth on Eurasian lynx mortality in the Białowieża Primeval Forest, Poland. Mamm Res 60, 3–8 (2015). https://doi.org/10.1007/s13364-014-0203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-014-0203-z