Abstract
Characterizing and elucidating structures is a commonplace and necessary activity in the pharmaceutical industry with mass spectrometry and NMR being the primary tools for analysis. Although many functional groups are readily identifiable, quaternary ammonium cations have proven to be difficult to unequivocally identify using these techniques. Due to the lack of an N–H bond, quaternary ammonium groups can only be detected in the 1H NMR spectra by weak signals generated from long-range 14N–H coupling, which by themselves are inconclusive evidence of a quaternary ammonium functional group. Due to their low intensity, these signals are frequently not detected. Additionally, ions cannot be differentiated in a mass spectrum as an M+ or [M + H]+ ion without prior knowledge of the compound’s structure. In order to utilize mass spectrometry as a tool for determining this functionality, ion cluster formation of quaternary ammonium cations and non-quaternary amines was studied using electrospray ionization. Several mobile phase modifiers were compared; however, the addition of small amounts of trifluoroacetic acid proved superior in producing characteristic and intense [M +2TFA]– clusters for compounds containing quaternary ammonium cations when using negative electrospray. By fragmenting this characteristic ion using CID, nearly all compounds studied could be unambiguously identified as containing a quaternary ammonium cation or a non-quaternary amine attributable to the presence (non-quaternary amine) or absence (quaternary ammonium cation) of the resulting [2TFA + H]– ion in the product spectra. This method of analysis provides a rapid, novel, and reliable technique for indicating the presence of quaternary ammonium cations in order to aid in structural elucidation.

ᅟ
Similar content being viewed by others
1 Introduction
Quaternary ammonium cations, also known as quats, are nitrogen-containing ions with four alkyl or aryl points of attachment to the nitrogen in order to yield a permanent positive charge on the molecule (structure NR4 +). They are often found with an anion in their salt form (called quaternary ammonium salts, quaternary ammonium compounds, or quaternary amines). For simplicity, both quaternary ammonium cations and quaternary ammonium salts will be collectively referred to as quats. Their counterparts, referred to here generally as non-quaternary amines or non-quats, are all other amine-containing compounds (primary, secondary, or tertiary amines) that do not also contain a quaternary ammonium functionality. Quats exist endogenously within various tissues of the body (such as the central nervous system) and play a vital role in numerous biochemical pathways [1, 2]. Additionally, they are frequently synthesized and routinely utilized in a variety of industries, with their uses ranging from personal care items such as antimicrobials [3–5] and antistatic agents [6, 7] to herbicides [8–11] and pharmaceuticals [12, 13].
Much work has been published on detecting and quantitating targeted quats in differing matrices [14–19]. Detection methods that require ion formation, such as mass spectrometry (MS), take advantage of the permanent positive charge already present on quaternary ammonium cations. The quat structure itself, however, has simultaneously hampered the use of MS in recognizing its presence in unknown compounds, as well as by the other prominent structure elucidation tool of nuclear magnetic resonance (NMR). As an N–H bond is not present in quats, 1H NMR can only provide information regarding the quaternary functional group via long-range quadrupolar coupling to 14N. These signals are frequently small and, therefore, may not be detected [20]. Although other modes of NMR may provide further information that may indicate the possible presence of a quat, they are not confirmatory or definitive [21, 22]. Additionally, mass spectrometry suffers from the inability to distinguish between the M+ ion of a quat and the [M + H]+ ion of a non-quat in a mass spectrum. Previously published work used liquid secondary ion mass spectrometry (LSIMS) coupled to a multi-step procedure and analysis in order to determine quaternary ammonium compounds [23]; however several functional groups complicated the recognition process and it could not be used as a universal method. Furthermore, LSIMS is no longer regularly employed.
Particularly for pharmaceutical impurity analysis, it would be highly desirable to have an unambiguous means for readily recognizing quats using common, commercially-available and widely-utilized instrumentation. Non-quat active pharmaceutical ingredients (APIs) have the ability to generate impurities with either quaternary ammonium cations or non-quaternary amines (similarly, quat APIs may form quat or non-quat impurities). When those impurities surpass the limits set by International Conference on Harmonization (ICH) guidance [24, 25], their structure must be ascertained. Also, crystal structure verification is impractical for use in routine structure elucidation, especially for low-level impurity analysis where impurities may either be present in a mixture or present in their pure form in sub-mg quantities. For these reasons, electrospray mass spectrometry (ESI-MS) was investigated for the identification of quats. Ion cluster formation of quats versus non-quats was studied using both positive and negative ESI-MS with mobile phases of varying composition (solvent and additives) in order to identify the most suitable conditions for producing characteristic ion clusters. This experiment was accomplished by comparing the presence and intensities of ions from quats and non-quats containing a variety of functional groups (in addition to their amine functionality). The chosen experimental combination of (–)ESI-MS with water:acetonitrile:trifluoroacetic acid (TFA) was used in further fragmentation studies utilizing the same compounds to assess the uniqueness of the product ion formation. Additionally, the application of this method to differentiate between impurities generated from an amine containing API for pharmaceutical structural analysis is presented.
2 Experimental
2.1 Materials
Eleven commercially available quats were studied. Isopropamide iodide (Fluka, ≥95%), choline chloride (99%), benzalkonium chloride, rocuronium bromide (≥98%), and betaine (≥98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA), cholin acetate (Fluka, BASF quality, ≥95%) from Sigma-Aldrich (Steinheim, Germany), L-carnitine (99%) and (2-chloroethyl)trimethylammonium chloride (also chlormequat chloride, 98%) from Aldrich (Milwaukee, WI, USA), neostigmine bromide (>99%) and D-tubocurarine chloride pentahydrate (>98%) from TCI America (Portland, OR, USA), and cocamidopropyl betaine (30%) from AK Scientific, Inc. (Union City, CA, USA).
Eight non-quaternary amines (seven commercially available and one BMS compound) were compared. Triethylamine (also TEA, >99.5%), amylamine (97%), cyclopentylamine (99%), (R)-(+)-propranolol hydrochloride (≥99%), (–)-isoproterenol (+)-bitartrate salt dihydrate, chlorhexidine (≥99.5%), and 3,4-dihydroxy-D-phenylalanine (also D-DOPA or dextrodopa) were purchased from Sigma-Aldrich (St. Louis, MO, USA). BMS-820836 was synthesized in house.
HPLC grade mobile phase solvents of acetonitrile and methanol were purchased from J.T. Baker (Phillipsburg, NJ, USA) and EMD Chemicals Inc. (Gibbstown, NJ, USA). Deionized water was purified using either a Millipore Milli-Q UV Plus (Billerica, MA, USA) or a Barnstead Nanopure Diamond (Thermo Scientific, Dubuque, IA, USA) operated at 18.2 MΩ. Mobile phase additives of trifluoroacetic acid (also TFA, HPLC grade), ammonium acetate (HPLC grade), and glacial acetic acid (HPLC grade) were obtained from J.T. Baker (Phillipsburg, NJ, USA), formic acid (Fluka, 98%) from Sigma-Aldrich (Steinheim, Germany), and ammonium chloride (≥99.5%), ammonium formate (≥99.995%), ammonium trifluoroacetate (Fluka, HPLC grade, ≥99.0%), and sodium iodide (≥99.99%) from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Samples
All samples were prepared individually in water at 10 mM with the exception of cyclopentamine, which was dissolved at 10 mM in acetonitrile:water (1:9 v/v) because of low solubility in water.
2.3 HPLC-MS
Liquid chromatography (LC) was performed using a Waters Acquity UPLC system consisting of a PDA Detector, Column Manager, Sample Manager, and Binary Solvent Manager (Waters Corporation, Milford, MA, USA). Samples were maintained at 5°C in the Sample Manager prior to being individually injected (1 μL) onto a heated column (35°C, Waters BEH C18, 2.1 × 50 mm, 1.7 μm) for gradient elution at 0.4 mL/min. Gradient elution consisted of a linear increase (0.00–3.00 min, 10%–100% B), an isocratic hold (3.00–3.45 min, 100% B), a sharp linear decrease (3.45–3.50 min, 100%–10% B), and an isocratic re-equilibration (3.50–5.00 min, 10% B). For mobile phase selection, seven sets of mobile phases (Table 1) were tested to compare the intensity and types of ion clusters formed.
Mass spectrometry was performed on a Waters Micromass Q-Tof Premier (Waters Corporation, Milford, MA, USA) equipped with an ESI source. For (+)ESI experiments, the capillary voltage was set at 3.5 kV, source temperature at 125°C, desolvation temperature at 350°C, cone voltage at 20.0 V, MS range of m/z 20–950, MS/MS range starting at m/z 10, precursor ions of M+ (quats) or [M + H]+ (non-quats), and collision energy at 20 V (also 10, 25, and 35 V for comparison). For (–)ESI experiments, the cone voltage was changed to 25.0 V, the MS/MS range started at m/z 20, precursor ions were [M +2TFA]– (quats) or [M + H +2TFA]– (non-quats), and the collision energy was changed to 10 V (also 5, 7, 8, and 20 V for comparison). For both polarities, the MS/MS precursor ions were fragmented using collision induced dissociation (CID) in the collision cell with argon gas. All instrumentation was controlled using MassLynx software ver. 4.1 (Waters Corporation, Milford, MA, USA).
2.4 Impurity Analysis
Samples of BMS-663068 (a non-quat synthesized in-house) were prepared at approximately 5 mg/mL in methanol:water (1:1 v/v). Liquid chromatography was performed on an Agilent 1100 Series system consisting of a QuatPump, ColCom, DAD, and 1200 Series HiP-ALS (Agilent Technologies, Santa Clara, CA, USA). Samples were individually injected (3–20 μL) on column (25°C, Waters XBridge BEH Phenyl, 4.6 × 150 mm, 3.5 μm) for gradient elution at 1.0 mL/min and UV detection at 302 nm. Gradient elution consisted of a linear increase (0.0–25.0 min, 0%–36% B), a linear increase (25.0–35.0 min, 36%–62% B), a sharp linear increase (35.0–36.0 min, 62%–100% B), an isocratic hold (36.0–40.0 min, 100% B), a sharp linear decrease (40.0–40.1 min, 100%–0% B), and an isocratic re-equilibration (40.1–47.0 min, 0% B). Mobile phase A was composed of 10 mM ammonium acetate in water:methanol (80:20 v/v) with mobile phase B containing 10 mM ammonium acetate in water:methanol:acetonitrile (5:20:75 v/v/v). For (–)ESI-MS experiments, 1 mM ammonium trifluoroacetate was added to each of the mobile phases.
Mass spectrometry was performed using a Thermo Scientific LTQ Orbitrap Discovery (Thermo Scientific, San Jose, CA, USA) equipped with an ESI source. For (+)ESI experiments, the spray voltage was set at 4.5 kV, capillary temperature at 275°C, capillary voltage at 41.5 V, tube lens at 116 V, MS range of m/z 125–2000, and the Orbitrap resolution at 30,000. For (–)ESI experiments, the capillary voltage was changed to 35.0 V and the tube lens to 100 V while utilizing the LTQ linear ion trap for mass analysis. All instrumentation was controlled using XCalibur software ver. 2.0.7 (Thermo Scientific, San Jose, CA, USA).
3 Results and Discussion
3.1 LC/MS Investigation of Cluster Formation
As quats contain a permanent positive charge, they are readily detected using (+)ESI; however, the M+ ion is non-characteristic and cannot be differentiated from an [M + H]+ ion in a mass spectrum. While a non-quat can readily form an [M – H]– ion using (–)ESI, a quat will require the addition of two negative charges in order to generate a charge of –1 [8, 10, 26]. Rather than forming a [M – 2H]– ion, we hypothesized that a quat would preferentially cluster with two anions (A) present in solution to form a [M +2A]– ion with its analogous non-quat favoring ionization as the [M – H]– ion (or not forming a negative ion) compared with a [M + H +2A]– ion. In order to produce the [M +2A]– cluster, an appropriate anion would be required, as not all anions would have the same propensity to form the cluster [27] (see example mass spectra of quat and non-quat in Supplementary Figure 1).
To make this methodology for quat recognition amenable to a multi-component analysis, reversed-phase (RP) gradient liquid chromatography was coupled to the mass spectrometer rather than utilizing constant infusion. A chromatographic separation would also assist in removing any possible ionization effects caused by counter-ions present in the sample solution. Since counter-ions dissociate from the compound in solution and should elute in the chromatographic void time under RP conditions, they should not be present when the analyte of interest elutes, assuming there is some retention of that analyte.
To prove the effectiveness of the hypothesis on quats, a range of compounds containing different functionalities were chosen, as listed in Table 2 (with structures shown in Supplementary Figure 2). Each of the quats was individually analyzed using (+)ESI, then (–)ESI with each of the mobile phase sets listed in Table 1. Under (+)ESI conditions, the M+ ion of each quat was observed as expected without the observance of any anion clusters. Although this ion is non-characteristic for quat determination, it is necessary for determining molecular weight information when the compound being analyzed is unknown. A quat ionized as M+ will have an m/z value 226 units greater in negative mode as [M +2TFA]–, whereas a non-quat ionized as [M + H]+ could possibly also have an m/z value 226 units greater in negative mode as [M + H +2TFA]–.
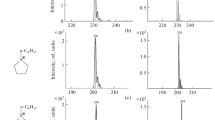
For all mobile phase sets tested using (–)ESI, quat ion clusters were formed, predominantly as the [M +2A]– cluster, although other variations such as [M – H + A]– were also observed, consistent with the hypothesis that negative ions from quats would be predominantly in the form of cluster ions. The resulting clusters for each mobile phase set are summarized in Table 3. When 0.05% TFA was added to the mobile phase, the anion for the ion cluster was very specific: A = TFA (where TFA is the deprotonated anion). For all other mobile phase sets tested, more than one type of anion cluster was observed. Ammonium formate and formic acid containing mobile phases produced [M +2formate]– clusters as well as [M +2TFA]– clusters. Similarly, ammonium chloride containing mobile phase produced both TFA and chloride anion clusters, mobile phases with ammonium acetate formed formate, TFA, and acetate clusters, with the addition of sodium iodide to the mobile phase yielding iodide, TFA, and formate clusters (no acetate clusters). Although TFA was only added to one set of mobile phases, it produced [M +2TFA]– clusters, often as the most dominant ion, for all combinations tested (see Figure 1). This effect was not due to mobile phase contamination as not only were the LC lines purged extensively between mobile phase changes but also the TFA containing mobile phases were used exclusively utilizing the A2/B2 lines of the Acquity system, with all others on the A1/B1 lines. Residual amounts of TFA are likely present on the LC components shared by both sets of lines as well as in the MS source chamber. When small amounts of TFA were directly added to the mobile phase, TFA clusters were formed exclusively; when only trace levels of TFA existed in the presence of other anions within the instrumental system, TFA clusters were still the dominantly formed ion cluster. Due to this superior ability of TFA to form the desired characteristic anion cluster, it was chosen as a mobile phase additive for the remainder of the experimentation. This capacity is consistent with the ability of TFA to act as a strong ion-pairing agent in liquid chromatography separations as well as the highly polar nature of the anion.
(–)ESI mass spectra of quats using non-TFA containing mobile phases. (a) Choline chloride using ammonium formate (with acetonitrile), (b) chlormequat chloride using formic acid, (c) neostigmine bromide using ammonium chloride, (d) benzalkonium chloride using ammonium formate (with methanol), (e) choline chloride using ammonium acetate
For comparison, non-quats containing various functional groups (see Table 2 and Supplementary Figure 2) were analyzed using the 0.05% TFA containing mobile phase conditions chosen above for quat analysis. Although the hypothesis that a quat would form a [M +2A]– ion with its analogous non-quat favoring ionization as the [M – H]– ion (or not forming a negative ion) compared with a [M + H +2A]– ion held true for all of the quats and several non-quat compounds, it did not hold true for all non-quats. Some of the non-quats also produced the cluster ion with two TFA molecules, demonstrating that the differentiation between quats and non-quats could not be performed by the appearance of cluster ions alone. As seen in Figure 2a, simple compounds, such as chlormequat chloride and amylamine, ionized as predicted. Chlormequat (quat) produced a large [M +2TFA]– cluster, whereas amylamine (non-quat) formed a dominant [2TFA + H]– cluster (although a very small [M + H +2TFA]– ion was also present). Carboxylic acid-containing compounds, such as carnitine (quat) and dextrodopa (non-quat), both generated non-discriminatory TFA clusters shown in Figure 2b. Carnitine’s base peak was a [M – H + TFA]– cluster, with its second most intense ion being the [M +2TFA]– cluster; however, dextrodopa primarily ionized as the [M + TFA]– cluster. As the dominant ion cluster of both compounds contained a single TFA, this complicated the recognition of a quat from a non-quat. The hypothesis also failed for compounds that contained multiple functional groups in addition to their amine group, such as cocamidopropyl betaine (quat) and chlorhexidine (non-quat). As shown in Figure 2c, both compounds produced analogous single and double TFA clusters.
(–)ESI mass spectra comparisons between quats and non-quats containing different functional groups. The mobile phase contains 0.05% TFA. (a) Simple structures, (b) contain carboxylic acid, (c) contain multiple groups. Quats: (a) chlormequat chloride, (b) carnitine, (c) cocamidopropyl betaine. Non-quats: (a) amylamine, (b) dextrodopa, (c) chlorhexidine
3.2 LC/MS/MS of TFA Clusters
The lack of a quat functional group can be inferred for some compounds because of their inability to ionize when using (–)ESI. Additionally, although they ionize in (–)ESI, some other non-quats can be readily identified as they fail to form with a double TFA cluster in the MS. For those compounds that do produce the expected cluster with (–)ESI, further confirmation is necessary. In these cases, LC/MS/MS of the [M +2TFA]– (or corresponding [M + H +2TFA]–) clusters is required in order to make a definitive determination of a quat versus a non-quat.
It was observed during the course of this investigation that the [M + H +2TFA]– cluster of non-quats produced an abundant fragment ion at m/z 227 when subjected to (–)ESI-LC/MS/MS. This fragment was absent when subjecting the analogous quat cluster to MS/MS (see Supplementary Figure 3), suggesting another approach for differentiating quats from non-quats. The m/z 227 ion is the [2TFA + H]– ion cluster, which is presumably formed by the dissociation of the two TFA anions associated with a protonated amine. In order to form this fragment cluster, a labile nitrogen proton must be present, which is present on non-quats but absent from quats. By using tandem mass spectrometry, the presence or absence of the m/z 227 fragment cluster can be used as an additional differentiating factor. For non-quaternary amines, the [2TFA + H]–fragment cluster is present in the product spectrum, whereas the [2TFA + H]– fragment cluster is absent for quats. A decision tree for identifying a quat versus a non-quat is diagrammed in Figure 3. Although it is not uncommon to see TFA ions or clusters present as background ions in a mass spectrum, it is important to note that the double TFA fragment cluster observed here in the MS/MS spectra is not merely a background ion but is an actual fragmentation product formed only when non-quats are fragmented (compared with quats). This unique identifying fragment ion is further confirmed by the peak present in the extracted ion chromatogram of the m/z 227 ([2TFA + H]–) ion, as shown in Supplementary Figure 3.
Of the 19 compounds studied, 11 were quats, eight were non-quats. Using the flow diagram in Figure 3, 100% of the non-quats were identified correctly as non-quaternary amine compounds. Although all of the quats analyzed formed an ion using (–)ESI, neither the non-quats TEA nor BMS-820836 ionized using (–)ESI. Additionally, all quats studied formed the [M +2TFA]– cluster (although the ion intensity varied greatly among compounds), whereas the non-quat D-DOPA did not form the [M + H +2TFA]– cluster. All remaining non-quats were identified by fragmenting the [M + H +2TFA]– cluster, noting the formation of the [2TFA + H]– fragment cluster in the product spectrum.
From the remaining 11 quats, 82% (9/11) were identified correctly as quats using the decision tree shown in Figure 3. Benzalkonium chloride, chlormequat chloride, choline chloride, choline acetate, neostigmine bromide, rocuronium bromide, isopropamide iodide, carnitine, and betaine were identified as quats as they did not form the [2TFA + H]– ion fragment cluster in the product spectrum when the [M +2TFA]– cluster was fragmented. Both cocamidopropyl betaine and tubocuraraine were misidentified as non-quaternary amines because of the observance of a small [2TFA + H]– fragment cluster in the product spectrum. The formation of the [2TFA + H]– fragment cluster may be due to the structures containing both a quat as well as a non-quat with a labile proton (see Supplementary Figure 2). As this paper grouped the tested compounds as either a quat or a non-quat, it may be more suitable to classify the compounds into three categories: quats, non-quats, and compounds containing both quaternary ammonium cations and non-quaternary amines with labile protons. Note that rocuronium, isopropamide, and neostigmine all contain both a quat and non-quat functionality in their structures; however, as the protons were not labile (or as labile), they were all identified correctly as quats.
3.3 Application: Impurity Analysis
While the general ability to specifically identify quats and non-quats is desirable, our intended focus is to accurately, easily, and rapidly discern chemical structures of impurities present in pharmaceuticals. This process can either be accomplished directly using the above-mentioned LC/MS/MS method or via a comparison of the ionizing behavior of related known compounds. Additionally, both techniques mentioned above can be applied to compounds containing an iminium cation, as shown below, because of the similarity in structure between quats and iminium cations. Both functionalities contain a nitrogen with four bonds to alkyl or aryl groups, thereby producing a permanent, positive charge on the molecule. This feature allows for differentiation between compounds containing quaternary nitrogens (quaternary ammonium cations and iminium cations) and non-quaternary amines. Both the decision tree and a comparative analysis were utilized in the impurity analysis of a pharmaceutical candidate, BMS-663068-03, when a previously undetected impurity was observed in an LC/UV chromatogram. The API, BMS-663068-03, is a non-quaternary amine that contains four tertiary amines and three imines (see Figure 4). The unidentified impurity (impurity 3) eluted closely after two previously identified isomers, impurity 1 (iminium ion that also contains four tertiary amines and two imines) and impurity 2 (non-quat that contains four tertiary amines and two imines) (structures shown in Figure 4). Accurate mass measurements of impurity 3 using (+)ESI confirmed impurity 3 to be another isomer of impurities 1 and 2 (Figure 5). The (+)ESI mass spectra of all three impurities were very similar and did not exhibit any distinguishing features; all three produced a dominant [M + H]+ ion with an additional less intense ion that is 22 Da more than the protonated ion, indicating that sodium adduction does not provide differentiation between quats and non-quats using (+)ESI. To determine if the structure contained a quaternary nitrogen, ammonium trifluoroacetate was added to the mobile phases in order to provide a TFA anion; the analytes were then subject to LC/MS analysis using (–)ESI. The amount of ammonium trifluoroacetate added to the mobile phases was small and, therefore, had a negligible impact on the chromatography. The known iminium impurity (impurity 1) formed the [M + H +2TFA]– cluster (the additional hydrogen is due to the zwitterionic structure of the compound) whereas both the non-quat API and known non-quat impurity (impurity 2) each formed a base peak [M + TFA]– cluster with an additional, smaller [M – H]– ion. Impurity 3 similarly formed a dominant [M + TFA]– cluster and a less intense [M – H]– ion, whereas the [M + H +2TFA]– cluster was not detected. Using the workflow diagrammed in Figure 3, impurity 3 was identified as a non-quaternary amine because the analyte had ionized using (–)ESI-MS; however, it did not form the [M + H +2TFA]– cluster ion. The impurity structure was later confirmed by comparison of the mass spectrum and LC retention time with those from the synthesized impurity compound.
LC/UV chromatogram of pharmaceutical impurity analysis application with corresponding mass spectra using (+)ESI and (–)ESI. (a) UV chromatogram, λ =302 nm, (b) (+)ESI mass spectrum of BMS-663068 (API), (c) (+)ESI mass spectrum of impurity 1, (d) (+)ESI mass spectrum of impurity 2, (e) (+)ESI mass spectrum of impurity 3, (f) (–)ESI mass spectrum of BMS-663068 (API), (g) (–)ESI mass spectrum of impurity 1, (h) (–)ESI mass spectrum of impurity 2, (i) (–)ESI mass spectrum of impurity 3. Note that the (+)ESI spectra were obtained using the Orbitrap mass analyzer, whereas the (–)ESI spectra were acquired using the linear ion trap of the instrument, hence the difference in mass accuracy shown
This non-quat designation is further supported by simply comparing the clusters formed by the other isomeric impurities (see Figure 5) and API using (–)ESI MS. The iminium cation formed a [M + H +2TFA]– ion cluster, whereas the known non-quats all formed a sizeable [M + TFA]– ion with a supplementary, less intense [M – H]– ion instead of the double TFA cluster. As the unknown impurity produced the same ionization pattern of an intense single TFA cluster along with a deprotonated ion, its structure can be inferred to be a non-quat. Similar impurity analyses are frequently encountered in the pharmaceutical industry; by employing the use of TFA in mobile phases with (–)ESI MS detection, this comparative technique can be a very powerful means for differentiating quats and non-quats without necessitating the full workflow diagramed in Figure 3. This comparison can also be used in conjunction with the method diagramed in the Figure 3 decision tree to either provide further support of the structural characterization provided or to assist in elucidation of compounds that contain more complicated structures (such as additional functional moieties).
4 Conclusions
The ion clusters of quaternary ammonium cations and non-quaternary amines were studied using positive and negative electrospray ionization in order to develop a method capable of identifying quat versus non-quat containing compounds using mass spectrometry alone. Although the M+ and [M + H]+ ions of unknown quats and non-quats, respectively, cannot be differentiated by viewing a mass spectrum taken using (+)ESI, differing ion clusters are observed in the mass spectra using (–)ESI and mobile phases containing an acid or salt modifier. The most intense and distinctive ion clusters were observed when TFA was included in the mobile phase composition, yielding [M +2TFA]– clusters for quats (and frequently [M +2TFA + H]– ion clusters for non-quats). Although we initially hypothesized that quats and non-quats would form different ion clusters simply using (–)ESI-MS with a modified mobile phase, the process to distinguish between the two is more complex. While quats always formed the [M +2TFA]–cluster under these ionization and mobile phase conditions, some non-quats either did not ionize or did not form a cluster containing two TFA anions, thereby making them easily distinguishable as non-quats. In order to definitively differentiate a quat from a non-quat, it was discovered that LC/MS/MS using (–)ESI with a TFA modified mobile phase was required. The [2TFA + H]– fragment cluster was always present in the product spectrum of the [M + H +2TFA]– cluster of non-quats, whereas quaternary ammonium cations did not typically produce this cluster when their [M +2TFA]– cluster was subject to fragmentation. The power and relevance of this identification process was further demonstrated in the pharmaceutical impurity analysis of BMS-663068-03, where a low-level impurity was correctly identified as a non-quat. By utilizing the rapid and novel method described here, (–)ESI mass spectrometry can be used to reliably determine the majority of quat containing compounds in order to assist in structural elucidation.
References
Jones, L.L., McDonald, D.A., Borum, P.R.: Acylcarnitines: role in brain. Prog. Lipid Res. 49, 61–75 (2010)
Sarter, M., Parikh, V., Howe, W.M.: Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat. Rev. 10, 383–390 (2009)
Ma, S., Izutani, N., Imazato, S., Chen, J.H., Kiba, W., Yoshikawa, R., Takeda, K., Kitagawa, H., Ebisu, S.: Assessment of bactericidal effects of quaternary ammonium-based antibacterial monomers in combination with colloidal platinum nanoparticles. Dental Mater. J. 31, 150–156 (2012)
Gottenbos, B., van der Mei, H.C., Klatter, F., Nieuwenhuis, P., Busscher, H.J.: In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 23, 1417–1423 (2002)
Lindstedt, M., Allenmark, S., Thompson, R.A., Edebo, L.: Antimicrobial activity of betaine esters, quaternary ammonium amphiphiles that spontaneously hydrolyze into nontoxic components. Antimicrob. Agents Chemother. 34, 1949–1954 (1990)
Sangermano, M., Foix, D., Kortaberria, G., Massimo, M.: Multifunctional antistatic and scratch resistant UV-cured acrylic coatings. Prog. Org. Coat. 76, 1191–1196 (2013)
Wçgrzyńska, J., Chlebicki, J.: Preparation, surface-active and antielectrostatic properties of multiple quaternary ammonium salts. J. Surfact. Deterg. 9, 221–226 (2006)
Evans, C.S., Startin, J.R., Goodall, D.M., Keely, B.J.: Formation of gas-phase clusters monitored during electrospray mass spectrometry: a study of quaternary ammonium pesticides. Rapid Commun. Mass Spectrom. 15, 1341–1345 (2001)
Castro, R., Moyano, E., Galceran, M.T.: Ion-pair liquid chromatography-atmospheric pressure ionization mass spectrometry for the determination of quaternary ammonium herbicides. J. Chromatogr. A 830, 145–154 (1999)
Milman, B.L.: Cluster ions of diquat and paraquat in electrospray ionization mass spectra and their collision-induced dissociation spectra. Rapid Commun. Mass Spectrom. 17, 1344–1349 (2003)
Whitehead Jr., R.D., Montesano, M.A., Jayatilaka, N.K., Buckley, B., Winnik, B., Needham, L.L., Barr, D.B.: Method for measurement of the quaternary amine compounds paraquat and diquat in human urine using high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 878, 2548–2553 (2010)
Storme, M.L., t’Kindt, R.S., Goeteyn, W., Reyntjens, K., Van Bocxlaer, J.F.: Quantitative determination of glycopyrrolate in human plasma by liquid chromatography-electrospray ionization mass spectrometry: the use of a volatile ion-pairing agent during both liquid–liquid extraction and liquid chromatography. J. Chromatogr. B 876, 24–30 (2008)
Schmidt, T., Widmer, R., Pfeiffer, A., Kaess, H.: Effect of the quaternary ammonium compound trospium chloride on 24 hour jejunal motility in healthy subjects. Gut 35, 27–33 (1994)
Li, X., Brownawell, B.J.: Analysis of quaternary ammonium compounds in estuarine sediments by LC-ToF-MS: very high positive mass defects of alkylamine ions as powerful diagnostic tools for identification and structural elucidation. Anal. Chem. 81, 7926–7935 (2009)
Falasca, S., Petruzziello, F., Kretz, R., Rainer, G., Zhang, Z.: Analysis of multiple quaternary ammonium compounds in the brain using tandem capillary column separation and high resolution mass spectrometric detection. J. Chromatogr. A 1241, 46–51 (2012)
Shackman, H.M., Shou, M., Cellar, N.A., Watson, C.J., Kennedy, R.T.: Microdialysis coupled on-line to capillary liquid chromatography with tandem mass spectrometry for monitoring acetylcholine in vivo. J. Neurosci. Methods 159, 86–92 (2007)
Hind, A.R., Bhargava, S.K., Cussis, P.G.: Quantitation of quaternary ammonium compounds using electrospray mass spectrometry. Anal. Chim. Acta. 377, 39–45 (1998)
Yang, W.-C., Adamec, J., Regnier, F.E.: Enhancement of the LC/MS analysis of fatty acids through derivatization and stable isotope coding. Anal. Chem. 79, 5150–5157 (2007)
Min, H.K., Kong, G., Moon, M.H.: Quantitative analysis of urinary phospholipids found in patients with breast cancer by nanoflow liquid chromatography-tandem mass spectrometry: II. Negative ion mode analysis of four phospholipid classes. Anal. Bioanal. Chem. 396, 1273–1280 (2010)
Luzhkov, V.B., Österberg, F., Acharya, P., Chattopadhyaya, J., Åqvist, J.: Computational and NMR study of quaternary ammonium ion conformations in solution. Phys. Chem. Chem. Phys. 4, 4640–4647 (2002)
Martin, G.J., Martin, M.L., Gouesnard, J.P.: 15N Chemical Shifts. In: NMR Basic Principles and Progress, Vol. 18, 15N-NMR Spectroscopy, 1st ed., pp. 75–186; Springer: Berlin, Heidelberg (1981)
Martin, G.E., Solntseva, M., Williams, A.J.: Applications of 15N NMR Spectroscopy in Alkaloid Chemistry. In: Fattorusso, E., Taglialatela-Scafati, O. (eds.) Modern Alkaloids: Structure, Isolation, Synthesis, and Biology, pp. 409–472. Wiley-VCH, Weinheim (2008)
Fisher, D.L., Moseley, M.A., Mullis, J.O., Norwood, D.L.: Recognition of quaternary ammonium compounds using mass spectrometry. Rapid Commun. Mass Spectrom. 8, 65–70 (1994)
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonized Tripartite Guideline Q3A (R2): Impurities in New Drug Substances (2006)
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonized Tripartite Guideline Q3B (R2): Impurities in New Drug Products (2006)
Lentz, N.B., Houk, R.S.: Negative ion mode electrospray ionization mass spectrometry study of ammonium-counter ion clusters. J. Am. Soc. Mass Spectrom. 18, 285–293 (2007)
Wang, G., Cole, R.B.: Effects of solvent and counterion on ion pairing and observed charge states of diquaternary ammonium salts in electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 7, 1050–1058 (1996)
Acknowledgments
The authors thank Chang-Ching Chan for sample preparation, as well as the Bristol-Myers Squibb Analytical and Bioanalytical Development management for the opportunity to conduct this research as part of an intra-departmental rotation program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
(PDF 100 kb)
Supplementary Figure 2
(PDF 128 kb)
Supplementary Figure 3
(PDF 127 kb)
Rights and permissions
About this article
Cite this article
Shackman, H.M., Ding, W. & Bolgar, M.S. A Novel Route to Recognizing Quaternary Ammonium Cations Using Electrospray Mass Spectrometry. J. Am. Soc. Mass Spectrom. 26, 181–189 (2015). https://doi.org/10.1007/s13361-014-1019-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-014-1019-4