Abstract
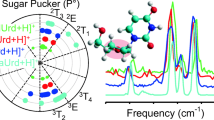
The gas-phase structures of alkali metal cation-cytosine complexes generated by electrospray ionization are probed via infrared multiple photon dissociation (IRMPD) action spectroscopy and theoretical calculations. IRMPD action spectra of five alkali metal cation–cytosine complexes exhibit both similar and distinctive spectral features over the range of ~1000–1900 cm-1. The IRMPD spectra of the Li+(cytosine), Na+(cytosine), and K+(cytosine) complexes are relatively simple but exhibit changes in the shape and shifts in the positions of several bands that correlate with the size of the alkali metal cation. The IRMPD spectra of the Rb+(cytosine) and Cs+(cytosine) complexes are much richer as distinctive new IR bands are observed, and the positions of several bands continue to shift in relation to the size of the metal cation. The measured IRMPD spectra are compared to linear IR spectra of stable low-energy tautomeric conformations calculated at the B3LYP/def2-TZVPPD level of theory to identify the conformations accessed in the experiments. These comparisons suggest that the evolution in the features in the IRMPD action spectra with the size of the metal cation, and the appearance of new bands for the larger metal cations, are the result of the variations in the intensities at which these complexes can be generated and the strength of the alkali metal cation-cytosine binding interaction, not the presence of multiple tautomeric conformations. Only a single tautomeric conformation is accessed for all five alkali metal cation–cytosine complexes, where the alkali metal cation binds to the O2 and N3 atoms of the canonical amino-oxo tautomer of cytosine, M+(C1).

ᇵ






Similar content being viewed by others
References
Crick, F.: Central dogma of molecular biology. Nature 227, 561–563 (1970)
Clarke, M.J.: Electrochemistry, synthesis, and spectra of pentaammineruthenium(III) complexes of cytidine, adenosine, and related ligands. J. Am. Chem. Soc. 100, 5068–5075 (1978)
Lippert, B., Schöllhorn, H., Thewalt, U.: Metal-stabilized rare tautomers of nucleobases. 1. Iminooxo form of cytosine: formation through metal migration and estimation of the geometry of the free tautomer. J. Am. Chem. Soc 108, 6616–6621 (1986)
Pichierri, F., Holthenrich, D., Zangrando, E., Lippert, B., Randaccio, L.: Metal-stabilized rare tautomers of nucleobases 5. Iminooxo tautomer of cytosine coordinated to Pt(II) with metal and nucleobase in syn and anti orientations. J. Biol. Inorg. Chem. 1, 439–445 (1996)
Müller, J., Zangrando, E., Pahlke, N., Freisinger, E., Randaccio, L., Lippert, B.: Affinity of the iminooxo tautomer anion of 1-methylcytosine in trans-[Pt(NH3)2(1-MeC-N4)2]2+ for heterometals. Chem. Eur. J. 4, 397–405 (1998)
Zamora, F., Kunsman, M., Sabat, M., Lippert, B.: Metal-stabilized rare tautomers of nucleobases. 6. Imino tautomer of adenine in a mixed-nucleobase complex of mercury(II). Inorg. Chem. 36, 1583–1587 (1997)
Arpalahti, J., Klika, K.D.: Platination of the exocyclic amino group of the adenine nucleobase by Pt(II) migration. Eur. J. Inorg. Chem. 8, 1199–1201 (1999)
Day, E.F., Crawford, C.A., Folting, K., Dunbar, K.R., Christon, G.: New metal-binding mode for adenine: A bidentate (N6, N7) bridging mode in the complex [Mo2(O2CCHF2)2(9-EtAH)2(MeCN)2](BF4)2•2MeCN. J. Am. Chem. Soc. 116, 9339–9340 (1994)
Velders, A.H., van der Geest, B., Kooijman, H., Spek, A.L., Haasnoot, J.G., Reedijk, J.: Ruthenium(III) coordination to the exocyclic nitrogen of 9-methyladenine and stabilisation of the rare imine tautomer by intramolecular hydrogen bonding. Eur. J. Inorg. Chem. 369−372 (2001)
Nei, Y.-W., Akinyemi, T.E., Kaczan, C.M., Steill, J.D., Berden, G., Oomens, J., Rodgers, M.T.: Infrared multiple photon dissociation action spectroscopy of sodiated uracil and thiouracils: Effects of thioketo-substitution on gas-phase conformation. Int. J. Mass Spectrom. 308, 191–202 (2011)
Yang, Z., Rodgers, M.T.: Tautomerization in the formation and collision-induced dissociation of alkali metal cation-cytosine complexes. Phys. Chem. Chem. Phys. 14, 4517–4526 (2012)
Watson, J.D., Crick, F.: Genetical implications of the structure of deoxyribonucleic acid. Nature 171, 964–967 (1953)
Topal, M.D., Fresco, J.R.: Complementary base pairing and the origin of substitution mutations. Nature 263, 285–289 (1976)
Šponer, J.: J., Hobza, P.: Bifurcated hydrogen bonds in DNA crystal structures. An ab initio quantum chemical study. J. Am. Chem. Soc. 116, 709–714 (1994)
Šponer, J., Hobza, P.: Nonplanar geometries of DNA bases. Ab initio second-order Moeller-Plesset study. J. Phys. Chem. 98, 3161–3164 (1994)
Hall, R.J., Burton, N.A., Hillier, I.H., Young, P.E.: Tautomeric equilibria in 2-hydroxypyridine and in cytosine. An assessment of density functional methods, including gradient corrections. Chem. Phys. Lett. 220, 129–132 (1994)
Sobolewski, A.L., Adamowicz, L.: Theoretical investigations of proton transfer reactions in a hydrogen bonded complex of cytosine with water. J. Chem. Phys. 102, 5708–5718 (1995)
Florián, J., Leszczyński, J., Johnson, B.G.: On the intermolecular vibrational modes of the guanine⋯cytosine, adenine–thymine and formamide–formamide H-bonded dimers. J. Mol. Struct. 349, 421–426 (1995)
Fülscher, M.P., Roos, B.O.: Theoretical study of the electronic spectrum of cytosine. J. Am. Chem. Soc. 117, 2089–2095 (1995)
Hobza, P., Šponer, J., Polásšek, M.: H-bonded and stacked DNA base pairs: Cytosine dimer. An ab initio second-order Moeller-Plesset study. J. Am. Chem. Soc. 117, 792–798 (1995)
Colominas, C., Luque, F.J., Orozco, M.: Tautomerism and protonation of guanine and cytosine. Implications in the formation of hydrogen-bonded complexes. J. Am. Chem. Soc. 118, 6811–6821 (1996)
Florián, J., Baumruk, V., Leszczyński, J.: IR and Raman spectra, tautomerism, and scaled quantum mechanical force fields of protonated cytosine. J. Phys. Chem. 100, 5578–5589 (1996)
Kwiatkowski, J.S., Leszczyński, J.: Molecular structure and vibrational IR spectra of cytosine and its thio and seleno analogues by density functional theory and conventional ab initio calculations. J. Phys. Chem. 100, 941–953 (1996)
Fogarasi, G.: High-level electron correlation calculations on some tautomers of cytosine. J. Mol. Struct. 413, 271–278 (1997)
Kobayashi, R.: A CCSD(T) study of the relative stabilities of cytosine tautomers. J. Phys. Chem. A 102, 10813–10817 (1998)
Russo, N., Toscano, M., Grand, A.: Lithium affinity for DNA and RNA nucleobases. The role of theoretical information in the elucidation of the mass spectrometry data. J. Phys. Chem. B 105, 4735–4741 (2001)
Russo, N., Toscano, M., Grand, A.: Bond energies and attachment sites of sodium and potassium cations to DNA and RNA nucleic acid bases in the gas phase. J. Am. Chem. Soc. 123, 10272–10279 (2001)
Fogarasi, G.: Relative stabilities of three low-energy tautomers of cytosine: A coupled cluster electron correlation study. J. Phys. Chem. A 106, 1381–1390 (2002)
Trygubenko, S.A., Bogdan, T.V., Rueda, M., Orozco, M., Luque, F.J., Šponer, J., Slavíček, P., Hobza, P.: Correlated ab initio study of nucleic acid bases and their tautomers in the gas phase, in a microhydrated environment and in aqueous solution. Part 1. Cytosine. Phys. Chem. Chem. Phys. 4, 4192–4203 (2002)
Yang, Z., Rodgers, M.T.: Theoretical studies of the unimolecular and bimolecular tautomerization of cytosine. Phys. Chem. Chem. Phys 6, 2749–2757 (2004)
Mazzuca, D., Marino, T., Russo, N., Toscano, M.: A theoretical study on tautomerization processes of dehydrated and monohydrated cytosine. THEOCHEM. 811, 161–167 (2007)
Szczesniak, M., Szczepaniak, K., Kwiatkowski, J.S., Kubulat, K., Person, W.B.: Matrix isolation infrared studies of nucleic acid constituents. 5. Experimental matrix-isolation and theoretical ab initio SCF molecular orbital studies of the infrared spectra of cytosine monomers. J. Am. Chem. Soc. 110, 8319–8330 (1988)
Brown, R.D., Godfrey, P.D., McNaughton, D., Pierlot, A.P.: Tautomers of cytosine by microwave spectroscopy. J. Am. Chem. Soc. 111, 2308–2310 (1989)
Monajjemi, M., Ghiasi, R., Sadjadi, M.A.S.: Metal-stabilized rare tautomers: N4 metalated cytosine (M = Li+, Na+, K+, Rb+ and Cs+), theoretical views. Appl. Organometal. Chem. 17, 635–640 (2003)
Lippert, B., Gupta. D.: Promotion of rare nucleobase tautomers by metal binding. Dalton Trans. 4619−4634 (2009)
Winter, M.: WebElements Periodic Table of the Elements | Cesium | biological information, WebElements (2012)
Valle, J.J., Eyler, J.R., Oomens, J., Moore, D.T., van der Meer, A.F.G., von Helden, G., Meijer, G., Hendrickson, C.L., Marshall, A.G., Blakney, G.T.: Free electron laser-Fourier transform ion cyclotron resonance mass spectrometry facility for obtaining infrared multiphoton dissociation spectra of gaseous ions. Rev. Sci. Instrum. 76, 023103–7 (2005)
Polfer, N.C., Oomens, J., Moore, D.T., von Helden, G., Meijer, G., Dunbar, R.C.: Infrared spectroscopy of phenylalanine Ag(I) and Zn(II) complexes in the gas phase. J. Am. Chem. Soc. 128, 517–525 (2006)
Polfer, N.C., Oomens, J.: Reaction products in mass spectrometry elucidated with infrared spectroscopy. Phys. Chem. Chem. Phys 9, 3804–3817 (2007)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery Jr., J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, Revision C.02. Gaussian, Inc, Wallingford (2004)
Foresman, J.B., Frisch, Æ.:Exploring Chemistry with Electronic Structures Methods, 2nd ed.; Gaussian: Pittsburg, 1996, p. 64.
Pople, J.A., Scott, A.P., Wong, M.W., Radom, L.: Scaling Factors for Obtaining Fundamental Vibrational Frequencies and Zero-Point Energies from HF/6-31G* and MP2/6-31G* Harmonic Frequencies. Isr. J. Chem. Soc. 33, 345–350 (1993)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision A.1. Gaussian, Inc, Wallingford (2009)
Weigend, F., Ahlrichs, R.: Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys 7, 3297–3305 (2005)
Leininger, T., Nicklass, A., Kuechle, W., Stoll, H., Dolg, M., Bergner, A.: The accuracy of the pseudopotential approximation: Non-frozen-core effects for spectroscopic constants of alkali fluorides XF (X = K, Rb, Cs). Chem. Phys. Lett. 255, 274–280 (1996)
Feller, D.: The role of databases in support of computational chemistry calculations. J. Comp. Chem. 17, 1571–1586 (1996)
Schuchardt, K.L., Didier, B.T., Elsethagen, T., Sun, L., Gurumoorthi, V., Chase, J., Li, J., Windus, T.L.: Basis set exchange: A community database for computational sciences. J. Chem. Inf. Model. 47, 1045–1052 (2007)
Armentrout, P.B., Chen, Y., Rodgers, M.T.: Metal cation dependence of interactions with amino acids: Bond energies of Cs+ to Gly, Pro, Ser, Thr, and Cys. J. Phys. Chem. A 116, 3989–3999 (2012)
McLean, A.D., Chandler, G.S.: Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, z = 11–18. J. Chem. Phys. 72, 5639–5648 (1980)
Hay, P.J., Wadt, W.R.: Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 82, 299–310 (1985)
Glendening, E.D., Feller, D., Thompson, M.A.: An ab initio investigation of the structure and alkali metal cation selectivity of 18-crown-6. J. Am. Chem. Soc. 116, 10657–10669 (1994)
Rodgers, M.T., Armentrout, P.B.: A critical evaluation of the experimental and theoretical determination of lithium cation affinities. Int. J. Mass Spectrom. 267, 167–182 (2007)
Armentrout, P.B., Austin, C.A., Rodgers, M.T.: Alkali metal cation interactions with 12-crown-4 in the gas phase: Revisited. Int. J. Mass Spectrom. 330/332, 16–26 (2012)
Wilson, R.G., Brewer, G.R.: Ion Beams with Applications to Ion Implantation. Wiley, New York (1973)
Salpin, J.-Y., Guillaumount, S., Tortajada, J., MacAleese, L., Lemaire, J., Maitre, P.: Infrared spectra of protonated uracil, thymine and cytosine. Chem. Phys. Chem. 8, 2235–2244 (2007)
Acknowledgments
The authors acknowledge financial support for this work by the National Science Foundation, grants OISE-0730072 and CHE-0911191. The authors also like to thank WSU C&IT for computer time. This work is part of the research program of FOM, which is financially supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO). The skillful assistance of the FELIX staff is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1090 kb)
Rights and permissions
About this article
Cite this article
Yang, B., Wu, R.R., Polfer, N.C. et al. IRMPD Action Spectroscopy of Alkali Metal Cation–Cytosine Complexes: Effects of Alkali Metal Cation Size on Gas Phase Conformation. J. Am. Soc. Mass Spectrom. 24, 1523–1533 (2013). https://doi.org/10.1007/s13361-013-0689-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-013-0689-7