Abstract
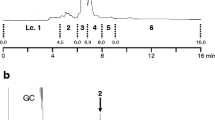
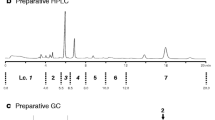
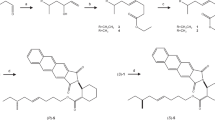
The grey pineapple mealybug, Dysmicoccus neobrevipes Beardsley, originally found on Hawaii and on Australasian islands, was recently discovered on a southwestern island (Ishigaki Island) of Japan. Because D. neobrevipes is known to attack many fruits and other crops, it is essential to establish a strategy to prevent the spread of this potential pest. Detection and monitoring by use of pheromone traps would provide important information about its distribution. The sex pheromone of D. neobrevipes has been isolated and identified as (E)-2-isopropyl-5-methylhexa-3,5-dien-1-yl acetate, although its absolute configuration was unknown. In this study, we achieved enantioselective synthesis of this compound by use of porcine pancreas lipase (PPL)-catalyzed acylation. Acetyl transfer from vinyl acetate to prochiral 2-isopropyl-1,3-propanediol in the presence of PPL in an organic solvent predominantly produced the (R) enantiomer of the monohydroxy acetate (86 % ee). In contrast, PPL-catalyzed hydrolysis of 2-isopropyl-1,3-diacetoxypropane in phosphate buffer yielded the (S) enantiomer of the monohydroxy acetate (75 % ee). Each enantiomer of the monohydroxy acetate was oxidized to a formyl acetate then coupled with a Wittig reagent, to produce the (R)-(−) and (S)-(+) pheromones. Analysis by gas chromatography with a chiral column and by polarimetry revealed the natural pheromone to be the (S)-(+) enantiomer. In a field trap experiment, the attractiveness of the pheromone produced by this route was equivalent to that of the pure (+) enantiomer (>99 % ee).


Similar content being viewed by others
References
Bando T (1998) Study on synthesis of biologically active molecules employing biocatalysts. Dissertation, Tokushima University
Ben-Dov Y (1994) A systematic catalogue of the mealybugs of the world (Insecta: Homoptera: Coccoidae: Pseudococcidae and Putoidae) with data on geographical distribution, host plants, biology and economic importance. Intercept, Andover 686 p
Ben-Dov Y (2014) Scale net. http://scalenet.info/validname/Dysmicoccus/neobrevipes/. Accessed 18 Aug 2014
Fand BB, Suroshe SS (2015) The invasive mealybug Phenacoccus solenopsis Tinsley, a threat to tropical and subtropical agricultural and horticultural production systems—a review. Crop Prot 69:34–43
Guanti G, Banfi L, Narisano E (1990) Enzymes in organic synthesis: remarkable influence of a π system on the enantioselectivity in PPL catalysed monohydrolysis of 2-substituted 1,3-diacetoxypropanes. Tetrahedron Asymmetr 1:721–724
Miller DR, Miller GL, Watson GW (2002) Invasive species of mealybugs (Hemiptera: Pseudococcidae) and their threat to US agriculture. Proc Entomol Soc Wash 104:825–836
Ramos Tombo GM, Schär HP, Fernandez i Busquets X, Ghisalba O (1986) Synthesis of both enantiomeric forms of 2-substituted 1,3-propanediol monoacetates starting from a common prochiral precursor, using enzymatic transformations in aqueous and in organic media. Tetrahedron Lett 27:5707–5710
Sugie H, Teshiba M, Narai Y, Tsutsumi T, Sawamura N, Tabata J, Hiradate S (2008) Identification of a sex pheromone component of the Japanese mealybug, Planococcus kraunhiae (Kuwana). Appl Entomol Zool 43:369–375
Tabata J (2009) Pest-monitoring systems by using synthetic pheromones. Plant Prot 63:781–783 (in Japanese)
Tabata J (2013) A convenient route for synthesis of 2-isopropyliden-5-methyl-4-hexen-1-yl butyrate, the sex pheromone of Planococcus kraunhiae (Hemiptera: Pseudococcidae), by use of β, γ- to α, β-double-bond migration in an unsaturated aldehyde. Appl Entomol Zool 48:229–232
Tabata J, Ichiki RT (2015) A new lavandulol-related monoterpene in the sex pheromone of the grey pineapple mealybug, Dysmicoccus neobrevipes. J Chem Ecol 41:194–201
Tabata J, Teshiba M, Hiradate S, Tsutsumi T, Shimizu N, Sugie H (2011) Cyclolavandulyl butyrate: an attractant for a mealybug parasitoid, Anagyrus sawadai (Hymenoptera: Encyrtidae). Appl Entomol Zool 46:117–123
Tabata J, Narai Y, Sawamura N, Hiradate S, Sugie H (2012) A new class of mealybug pheromones: a hemiterpene ester in the sex pheromone of Crisicoccus matsumotoi. Naturwissenschaften 99:567–574
Tabata J, Teshiba M, Shimizu N, Sugie H (2015) Mealybug mating disruption by a sex pheromone derived from lavender essential oil. J Essent Oil Res. doi:10.1080/10412905.2015.1007219
Tanaka H, Uesato T (2012) New records of some potential pest mealybugs (Hemiptera: Coccoidea: Pseudococcidae) in Japan. Appl Entomol Zool 47:413–419
Teshiba M, Shimizu N, Sawamura N, Narai Y, Sugie H, Sasaki R, Tabata J, Tsutsumi T (2009) Use of a sex pheromone to disrupt the mating of Planococcus kraunhiae (Kuwana) (Hemiptera: Pseudococcidae). Jpn J Appl Entomol Zool 53:173–180 (in Japanese with English summary)
Teshiba M, Sugie H, Tsutsumi T, Tabata J (2012) A new approach for mealybug management: recruiting an indigenous, but ‘non-natural’ enemy for biological control using an attractant. Entomol Exp Appl 142:211–215
Yasuhara F, Yamaguchi S, Kasai R, Tanaka O (1986) Assignment of absolute configuration of 2-substituted-1-propanols by 1H NMR spectroscopy. Tetrahedron Lett 27:4033–4034
Zada A, Dunkelblum E (2006) A convenient resolution of racemic lavandulol through lipase-catalyzed acylation with succinic anhydride: simple preparation of enantiomerically pure (R)-lavandulol. Tetrahedron Asymmetr 17:230–233
Acknowledgments
We appreciate the help of Drs H. Sugie and S. Hiradate (NIAES) with chemical analyses. J.T. acknowledges a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (no. 25871089).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabata, J., Ohno, S. Enantioselective synthesis of the sex pheromone of the grey pineapple mealybug, Dysmicoccus neobrevipes (Hemiptera: Pseudococcidae), for determination of the absolute configuration. Appl Entomol Zool 50, 341–346 (2015). https://doi.org/10.1007/s13355-015-0337-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-015-0337-8