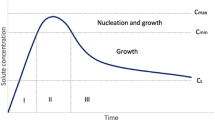
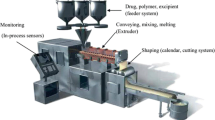
Abstract
Solid dispersion has emerged as a method of choice and has been extensively investigated to ascertain the in vivo improved performance of many drug formulations. It generally involves dispersion of drug in amorphous particles (clusters) or in crystalline particles. Comparatively, in the last decade, amorphous drug–polymer solid dispersion has evolved into a platform technology for delivering poorly water-soluble small molecules. However, the success of this technique in the pharmaceutical industry mainly relies on different drug–polymer attributes like physico-chemical stability, bioavailability and manufacturability. The present review showcases the efficacy of amorphous solid dispersion technique in the research and evolution of different drug formulations particularly for those with poor water soluble properties. Apart from the numerous mechanisms of action involved, a comprehensive summary of different key parameters required for the solubility enhancement and their translational efficacy to clinics is also emphasized.





Similar content being viewed by others
References
Baird J, Taylor L. Evaluation of amorphous SD properties using thermal analysis techniques. Adv Drug Deliv Rev. 2012;64:396–421.
Lipinski C, Lombardo F, Dominy B, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;46:3–26.
Mooter G. The use of amorphous SDs: a formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today Technol. 2011;9:79–85.
Kawabata Y, Wada K, Nakatani M, et al. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420:1–10.
Chau L, Chulhun P, Beom J. Current trends and future perspectives of SDs containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85:799–813.
Vasconcelos T, Sarmento B, Costa P. SDs as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12:1068–75.
Vasanthavada M, Tong W, Joshi Y, et al. Phase behaviour of amorphous molecular dispersions I: determination of the degree and mechanism of solid solubility. Pharm Res. 2004;21:1598–606.
Potta S, Minemi S, Nukala N, et al. Development of solid lipid nanoparticles for enhanced solubility of poorly soluble drugs. J Biomed Nanotechnol. 2010;6:634–40.
Rautio J, Kumpulainen H, Heimbach T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–70.
Pokharkar V, Mandpe L, Padamwar M, et al. Development, characterization and stabilization of amorphous form of a low Tg drug. Powder Technol. 2006;167:20–5.
Surampalli G, Sabbani P, Nanjwade K, et al. Amorphous SD method for improving oral bioavailability of poorly water-soluble drugs. J Pharm Res. 2013;6:476–80.
Rashid R, Kim D, Din F, Mustapha O, et al. Effect of hydroxypropylcellulose and Tween 80 on physicochemical properties and bioavailability of ezetimibe-loaded solid dispersion. Carbohydr Polym. 2015;130:26–31.
Tuong N, Ha-Lien P, Van Tran T, et al. Development of a modified-solid dispersion in an uncommon approach of melting method facilitating properties of a swellable polymer to enhance drug dissolution. Int J Pharm. 2015;484:228–34.
Onoue S, Terasawa N, Nakamura T, et al. Biopharmaceutical characterization of nanocrystalline SD of coenzyme Q10 prepared with cold wet-milling system. Eur J Pharm Sci. 2014;53:118–25.
Liu H, Ilevbare GA, Cherniawski BP, et al. Synthesis and structure–property evaluation of cellulose ω-carboxyesters for amorphous SDs. Carbohydr Polym. 2014;100:116–25.
Zhang Z, Chen Y, Deng J, et al. SD of berberine–phospholipid complex/TPGS 1000/SiO2: preparation, characterization and in vivo studies. Int J Pharm. 2014;465:306–16.
Onoue S, Nakamura T, Uchida A, Ogawa K, et al. Physicochemical and biopharmaceutical characterization of amorphous solid dispersion of nobiletin, a citrus polymethoxylated flavone, with improved hepatoprotective effects. Eur J Pharm Sci. 2013;49(4):453–60.
Ormes J, Zhang D, Chen A, et al. Design of experiments utilization to map the processing capabilities of a micro-spray dryer: particle design and throughput optimization in support of drug discovery. Pharm Dev Technol. 2013;18:121–9.
Martins R, Siqueira S, Freitas L. Spray congealing of pharmaceuticals: study on production of SDs using Box–Behnken design. Drying Technol. 2012;30:935–45.
Paudel G, Mooter D. Influence of solvent composition on the miscibility and physical stability of Naproxen/PVP K 25 SDs prepared by co-solvent spray-drying. Pharm Res. 2012;29:251–70.
Baret L, Voorpoles J, Kieken F. Powder for reconstitution. US. 2011;20110082161.
Kiser P, Gupta K. Linear order release polymer. US. 2011;2011004507.
Tiwari R, Janakiraman K, Agarwal R, et al. Solid dosage forms of HIV protease inhibitors. US. 2011;20110034489.
Besse J, Laurence B, Pournin J. Solid orodispersible and/or dispersible composition, without an excipient of known effect and its process of preparation. US. 2009;20090110725 .
Patel K, Pillai R. SD compositions. US. 2008;7423004.
Bedrosian C. Therapeutic methods. US. 2007;20070185150.
Terracol D, Duclos R. Process for the production of dry pharmaceutical forms and the thus obtained pharmaceutical compositions. US. 2000;6027747.
Fort J, Krill S, Law D, et al. SD pharmaceutical formulations. US. 1997;7364752.
Nakamichi K, Izumi S, Yasuura H. Method of manufacturing SD. US. 1995;5456923.
Nakano M, Uemura T, Morizane S, et al. Method of producing a SD of the sparingly water-soluble drug nilvadipine. US. 1994;5340591.
Baudier P, Boeck D, Fossion A. Novel galenic forms of verapamil, their preparation and medicines containing said novel galenic forms. US. 1989;4859469.
Riegelman S, Chiou W. Increasing the absorption rate of insoluble drugs. US. 1979;4151273.
Mayersohn M, Gibaldi M. New method of SD for increasing dissolution rates. J Pharm Sci. 1966;55:1323–24.
Tachibani T, Nakamura A. A method for preparing aqueous colloidal dispersion of organic materials by using water soluble polymers: dispersion of beta-carotene by polyvinyl pyrrolidone. Colloid Polym Sci. 1965;203:130–3.
Levy G. Effect of particle size on dissolution and gastrointestinal absorption rates of pharmaceuticals. Am J Pharm Sci. 1963;135:78–92.
Sekiguchi K, Obi N. Studies on absorption of eutectic mixtures. A comparison of the behavior of eutectic mixtures of sulphathiazole and that of ordinary sulphathiazole in man. Chem Pharm Bull. 1961;9:866–72.
Li B, Liu H, Amin M, et al. Enhancement of naringenin solution concentration by solid dispersion in cellulose derivative matrices. Cellulose. 2013;20(4):2137–49.
Satomi O, Suzuki H, Kojo Y, et al. Self-micellizing SD of cyclosporine A with improved dissolution and oral bioavailability. Eur J Pharm Sci. 2014;62:16–22.
Bhatnagar P, Dhote V, Mahajan SC, Mishra PK. Solid dispersion in pharmaceutical drug development: from basics to clinical applications. Curr Drug Deliv. 2014;11:155–71.
van Hoogevest P, Liu X, Fahr A. Drug delivery strategies for poorly water-soluble drugs: The industrial perspective. Expert Opin Drug Deliv. 2011;8:1481–500.
Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4:403–16.
Mooter G, Weuts I, Ridder T, et al. Evaluation of Inutec SP1 as a new carrier in the formulation of SDs for poorly soluble drugs. Int J Pharm. 2006;316:1–6.
Craig D. The mechanisms of drug release from SDs in water soluble polymers. Int J Pharm. 2002;231:131–44.
Leuner C, Dressman J. Improving drug solubility for oral delivery using SDs. Eur J Pharm Biopharm. 2000;50:47–60.
Sekiguchi K, Obi N. Studies on absorption of eutectic mixtures. Studies on Absorption of Eutectic Mixture. II. Absorption of Fused Conglomerates of Chloramphenicol and Urea in Rabbits. Chem Pharm Bull. 1964;12:134–44.
Van Drooge D, Hinrichs W, Visser M, et al. Characterization of the molecular distribution of drugs in glassy SDs at the nano-meter scale, using differential scanning calorimetry and gravimetric water vapour sorption techniques. Int J Pharm. 2006;310:220–9.
Zajc N, Obreza A, Bele M. Physical properties and dissolution behaviour of nifedipine/mannitol SDs prepared by hot melt method. Int J Pharm. 2005;291:51–8.
Tanaka N, Imai K, Okimoto K, et al. Development of novel sustained-release system, disintegration controlled matrix tablet (DCMT) with SD granules of nilvadipine (II): in vivo evaluation. J Control Release. 2006;112:51–6.
Urbanetz N. Stabilization of SDs of nimodipine and polyethylene glycol 2000. Eur J Pharm Sci. 2006;28:67–76.
Chauhan H, Hui-Gu C, Atef E. Correlating the behavior of polymers in solution as precipitation inhibitor to its amorphous stabilization ability in SDs. J Pharm Sci. 2013;102:1924–35.
Crowley M, Zhang F, Repka M, et al. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev Ind Pharm. 2007;33:909–26.
Vilhelmsen T, Eliasen H, Schaefer T. Effect of a melt agglomeration process on agglomerates containing SDs. Int J Pharm. 2005;303:132–42.
Lim H, Balakrishnan P, Hwang D, et al. Development of novel sibutramine base-loaded SD with gelatin and HPMC: physicochemical characterization and pharmacokinetics in beagle dogs. Int J Pharm. 2010;397:225–30.
Rodriguez J, Torre-Iglesias P, Vegas-Sánchez M, et al. Changed crystallinity of mebendazole SD: improved anthelmintic activity. Int J Pharm. 2011;403:23–8.
Bley H, Fussnegger B, Bodmeier R. Characterization and stability of SDs based on PEG/polymer blends. Int J Pharm. 2010;390:165–73.
Miyazaki T, Aso Y, Yoshioka S, et al. Differences in crystallization rate of nitrendipine enantiomers in amorphous SDs with HPMC and HPMCP. Int J Pharm. 2011;407:111–8.
Yunxia BI, Rahman A, David J. Solid dispersion of poorly soluble compounds comprising crospovidone and at least one water-soluble polymer. WO. 2013;2013040187 A.
Fort J, Krill S, Law D, et al. Solid dispersion pharmaceutical formulations. US. 2008;7364752 B1.
Ali W, Williams A, Rawlinson C. Stochiometrically governed molecular interactions in drug: poloxamer SDs. Int J Pharm. 2010;391:162–8.
Van Drooge D, Hinrichs W, Frijlink H. Anomalous dissolution behaviour of tablets prepared from sugar glass-based SDs. J Control Release. 2004;97:441–52.
Joshi H, Tejwani R, Davidovich M, et al. Bioavailability enhancement of a poorly water-soluble drug by SD in polyethylene glycol–polysorbate 80 mixture. Int J Pharm. 2004;269:251–8.
Maniruzzaman M, Boateng J, Bonnefille M, et al. Taste masking of paracetamol by hot-melt extrusion: an in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2012;80:433–42.
Saerens L, Dierickx L, Lenain B, et al. Raman spectroscopy for the in-line polymer–drug quantification and solid state characterization during a pharmaceutical hot-melt extrusion process. Eur J Pharm Biopharm. 2011;77:158–63.
Ghebremeskel A, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing SDs of poorly soluble API: selection of polymer–surfactant combinations using solubility parameters and testing the processability. Int J Pharm. 2007;328:119–29.
Parve B, Teli B, Birajdar A. Solid dispersions: an overview on solubility enhancement of poorly water soluble drugs. Int J Pharm Bio Sci. 2014;5:7–25.
Martin N, Pichler A, Richter F et al. Solid dispersion comprising amorphous lorcaserin hydrochloride. WO. 2014;2014135545 A1.
Passerini N, Albertini B, González M, et al. Preparation and characterisation of ibuprofen–poloxamer 188 granules obtained by melt granulation. Eur J Pharm Sci. 2002;15:71–8.
Verhoeven E, Beer T, Schacht E, et al. Influence of polyethylene glycol/polyethylene oxide on the release characteristics of sustained-release ethylcellulose mini-matrices produced by hot-melt extrusion: in vitro and in vivo evaluations. Eur J Pharm Biopharm. 2009;72:463–70.
Szuts A, Láng P, Ambrus R, et al. Applicability of sucrose laurate as surfactant in SDs prepared by melt technology. Int J Pharm. 2011;410:107–10.
Won D, Kim M, Lee S, et al. Improved physicochemical characteristics of felodipine SD particles by supercritical anti-solvent precipitation process. Int J Pharm. 2005;301:199–208.
Cui F, Yang M, Jiang Y, et al. Design of sustained-release nitrendipine microspheres having SD structure by quasi-emulsion solvent diffusion method. J Control Release. 2003;91:375–84.
Mogalian E, Oliyai R, Stefanidis D, et al. Solid dispersion formulation of an antiviral compound. US. 2014;20140212487 A1.
Martins R, Siqueira S, Freitas L. Spray congealing of pharmaceuticals: study on production of SDs using Box–Behnken design. Drying Technol. 2012;30:935–45.
Rein Richard H. Cryogenic cooling. US. 1968;3416977 A.
Chauhan B, Shimpi S, Paradkar A. Preparation and evaluation of glibenclamide-polyglycolized glycerides SDs with silicon dioxide by spray drying technique. Eur J Pharm Sci. 2005;26:219–30.
Yu DG, Zhu LM, White K, et al. Electrospun nanofiber based drug delivery systems. Health. 2009;1:67–75.
Hu J, Johnston K, Williams R. Spray freezing into liquid (SFL) particle engineering technology to enhance dissolution of poorly water soluble drugs: organic solvent versus organic/aqueous co-solvent systems. Eur J Pharm Sci. 2003;20:295–303.
Purvis T, Mattucci M, Crisp M, et al. Rapidly dissolving repaglinide powders produced by the ultra-rapid freezing process. AAPS Pharm Sci Tech. 2007;8:52–60.
Mura P, Moyano J, Rodriguez M, et al. Characterization and dissolution properties of ketoprofen in binary and ternary SDs with polyethylene glycol and surfactants. Drug Dev Ind Pharm. 2005;31:425–34.
Bhattacharya S, Suryanarayanan R. Local mobility in amorphous pharmaceuticals—characterization and implications on stability. J Pharm Sci. 2009;98:2935–53.
Duddu S, Sokoloski T. Dielectric analysis in the characterization of amorphous pharmaceutical solids. 1. Molecular mobility in poly (vinyl pyrrolidone)-water systems in the glassy state. J Pharm Sci. 1995;84:773–6.
Goddeeris C, Willems T, Houthoofd K, et al. Dissolution enhancement of the anti-HIV drug UC 781 by formulation in a ternary SD with TPGS 1000 and Eudragit E100. Eur J Pharm Biopharm. 2008;70:861–8.
Ohara T, Kitamura S, Kitagawa T, et al. Dissolution mechanism of poorly water-soluble drug from extended release SD system with ethylcellulose and hydroxyl propyl methyl cellulose. Int J Pharm. 2005;302:95–102.
Iqbal Z, Babar A, Ashraf M. Controlled-release naproxen using micronized ethyl cellulose by wet-granulation and solid-dispersion method. Drug Dev Ind Pharm. 2002;28:129–34.
Cai Z, Lei X, Lin Z, et al. Preparation and evaluation of sustained-release solid dispersions co-loading gastrodin with born eolasan oral brain-targeting enhancer. Acta Pharm Sinica B. 2014;4:86–93.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60.
Moes J, Koolen S, Huitema A, et al. Development of an oral solid dispersion formulation for use in low-dose metronomic chemotherapy of paclitaxel. Eur J Pharm Biopharm. 2013;83:87–94.
Moes JJ, Koolen SL, Huitema AD, et al. Pharmaceutical development and preliminary clinical testing of an oral solid dispersion formulation of docetaxel (ModraDoc001). Int J Pharm. 2011;420:244–50.
Tevaarwerk AJ, Holen KD, Alberti DB, et al. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin Cancer Res. 2009;15:1460–5.
Aboelwafa AA, Fahmy RH. A pilot human pharmacokinetic study and influence of formulation factors on orodispersible tablet incorporating meloxicam solid dispersion using factorial design. Pharm Dev Technol. 2012;17:1–14.
Kolasinac N, Kachrimanis K, Homsek M, et al. Solubility enhancement of desloratadine by SD in poloxamers. Int J Pharm. 2012;436:161–70.
Muhrer G, Meier U, Fusaro F, et al. Use of compressed gas precipitation to enhance the dissolution behaviour of a poorly water-soluble drug: generation of drug microparticles and drug–polymer SDs. Int J Pharm. 2006;308:69–83.
Tran T, Tran P, Lim J, et al. Physicochemical principles of controlled release SD containing a poorly water soluble drug. Ther Deliv. 2010;1:51–62.
Pouton C. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29:278–87.
Moschwitzer J. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453:142–56.
Yoshioka M, Hancock B, Zografi G. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J Pharm Sci. 1994;83:1700–5.
Vyazovkin S, Dranca I. Physical stability and relaxation of amorphous indomethacin. J Phys Chem B. 2005;109:18637–644.
Serajuddin A. SD of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88:1058–66.
Ahuja N, Katare O, Singh B. Studies on dissolution enhancement and mathematical modelling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur J Pharm Biopharm. 2007;65:26–38.
Ayenew Z, Paudel A, Van den Mooter A. Can compression induce demixing in amorphous SDs? A case study of naproxen–PVP K25. Eur J Pharm Biopharm. 2012;81:207–13.
Waard H, Hinrichs W, Visser M, et al. Unexpected differences in dissolution behaviour of tablets prepared from SDs with a surfactant physically mixed or incorporated. Int J Pharm. 2008;349:66–73.
Beg S, Swain S, Rizwan M, Irfanuddin M, et al. Bioavailability enhancement strategies: basics, formulation approaches and regulatory considerations. Curr Drug Deliv. 2011;8:691–702.
Vasanthavada M, Tong W, Joshi Y, et al. Phase behaviour of amorphous molecular dispersions II: role of hydrogen bonding in solid solubility and phase separation kinetics. Pharm Res. 2005;22:440–8.
Chiou W, Riegelman S. Preparation and dissolution characteristics of several fast-releases SDs of griseofulvin. J Pharm Sci. 1969;58:1505–510.
Conflict of interest
The authors state no conflict of interest and have received no payment in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, D.K., Dhote, V., Bhargava, A. et al. Amorphous solid dispersion technique for improved drug delivery: basics to clinical applications. Drug Deliv. and Transl. Res. 5, 552–565 (2015). https://doi.org/10.1007/s13346-015-0256-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-015-0256-9