Abstract
Objective
To examine long-term efficacy/safety of ipragliflozin, a sodium–glucose cotransporter 2 inhibitor, added to ongoing insulin therapy in Japanese patients with type 2 diabetes.
Methods
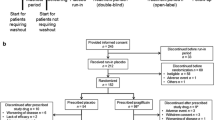
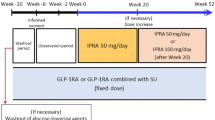
We conducted a 36-week, open-label extension of ipragliflozin therapy following a 16-week, randomized, placebo-controlled, double-blind period (treatment periods II and I, respectively). Prior to the open-label period, patients taking insulin with/without a dipeptidyl peptidase-4 (DPP-4) inhibitor were randomized to receive placebo or 50 mg once-daily ipragliflozin. Oral antidiabetic drugs other than DPP-4 inhibitors were discontinued 4 weeks before screening. Following treatment period I, all patients received open-label ipragliflozin 50 mg, with the possibility of a dose increase to 100 mg at week 24 if HbA1c was ≥ 7.0% at week 20. Efficacy endpoints were changes in HbA1c, fasting plasma glucose (FPG), self-monitored blood glucose, bodyweight, and metabolic hormones. Drug-related treatment-emergent adverse events (TEAEs) were monitored for safety.
Results
Of 175 patients randomized to ipragliflozin, 168 entered treatment period II, 121 (69%) of whom completed this period. The mean ± standard deviation changes in HbA1c, FPG, and bodyweight from baseline (start of treatment period I) to the end of treatment were − 0.83 ± 0.72%, − 31.5 ± 41.2 mg/dL, and − 1.34 ± 1.80 kg, respectively. Between weeks 8 and 32, HbA1c was lower in patients taking a DPP-4 inhibitor than in those without. The most common drug-related TEAE was hypoglycemia; no drug-related TEAEs not already reported for ipragliflozin were observed.
Conclusions
Ipragliflozin was well tolerated, effective, and reduced bodyweight over a period of 52 weeks in patients treated with insulin with/without a DPP-4 inhibitor.
Clinicaltrials.gov identifier
NCT02175784





Similar content being viewed by others
References
Lovre D, Fonseca V. Benefits of timely basal insulin control in patients with type 2 diabetes. J Diabetes Complications. 2015;29:295–301.
Pettus J, Santos Cavaiola T, Tamborlane WV, et al. The past, present, and future of basal insulins. Diabetes Metab Res Rev. 2016;32:478–96.
Wu T, Betty B, Downie M, et al. Practical guidance on the use of premix insulin analogs in initiating, intensifying, or switching insulin regimens in type 2 diabetes. Diabetes Ther. 2015;6:273–87.
Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin. 2011;27(Suppl 3):13–20.
Monami M, Ragghianti B, Zannoni S, et al. Identification of predictors of response to basal insulin and DPP-4 inhibitors in patients with type 2 diabetes failing to other therapies. Acta Diabetol. 2016;53:35–40.
Kashiwagi A, Kazuta K, Goto K, et al. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17:304–8.
Kashiwagi A, Kazuta K, Yoshida S, et al. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:382–91.
Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–60.
Ishihara H, Yamaguchi S, Nakao I, et al. Efficacy and safety of ipragliflozin as add-on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a multi-centre, randomized, placebo-controlled, double-blind study. Diabetes Obes Metab. 2016;18:1207–16.
Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295–303.
Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond). 2008;32(Suppl 7):S13–8.
Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–48.
Wilding JP, Woo V, Rohwedder K, et al. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–36.
Inagaki N, Harashima S, Maruyama N, et al. Efficacy and safety of canagliflozin in combination with insulin: a double-blind, randomized, placebo-controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:89.
Suzuki K, Mitsuma Y, Sato T, et al. Comparison of combined tofogliflozin and glargine, tofogliflozin added to insulin, and insulin dose-increase therapy in uncontrolled type 2 diabetes. J Clin Med Res. 2016;8:805–14.
Araki E, Onishi Y, Asano M, et al. Efficacy and safety of dapagliflozin in addition to insulin therapy in Japanese patients with type 2 diabetes: results of the interim analysis of 16-week double-blind treatment period. J Diabetes Investig. 2016;7:555–64.
Araki E, Onishi Y, Asano M, et al. Efficacy and safety of dapagliflozin over 1 year as add-on to insulin therapy in Japanese patients with type 2 diabetes: the DAISY (Dapagliflozin Added to patients under InSulin therapY) trial. Diabetes Obes Metab. 2016;19:562–70.
Acknowledgements
The authors wish to thank all of the investigators involved in this trial, as well as Nicholas Smith, PhD, and William Ng, MB, BS, PhD, of Edanz Medical Writing for providing medical writing services.
Funding
This study was sponsored by Astellas Pharma Inc. Medical writing and editorial support was funded by Astellas Pharma Inc. and provided by Dr. Nicholas D. Smith and Dr. William Ng (Edanz Medical Writing) and Elsevier/ELMCOM™.
Author information
Authors and Affiliations
Contributions
HI, SA, and TS contributed to study design, data analysis, and writing of the manuscript; SY and IN contributed to study design, study conduct, data collection and analysis, and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
HI has served on the scientific advisory board of Astellas Pharma Inc.; received lecture or consulting fees from Astellas Pharma Inc., MSD, Sanofi, Mitsubishi Tanabe Pharma, Boehringer Ingelheim Japan, and Novartis Pharma; and received grants/research support from Astellas Pharma Inc., Ono Pharmaceutical, Boehringer Ingelheim Japan, AstraZeneca, Sanofi, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Daiichi-Sankyo, Novo Nordisk Pharma, Kyowa Hakko Kirin, and MSD. SY, IN, SA, and TS are employees of Astellas Pharma Inc., Japan.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent or substitute for it was obtained from all patients for being included in the study. The study was approved by the institutional review board at each participating site.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ishihara, H., Yamaguchi, S., Nakao, I. et al. Efficacy and safety of ipragliflozin as add-on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a 36-week, open-label extension of a 16-week, randomized, placebo-controlled, double-blind study. Diabetol Int 10, 37–50 (2019). https://doi.org/10.1007/s13340-018-0359-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-018-0359-x