Abstract
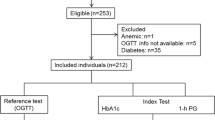
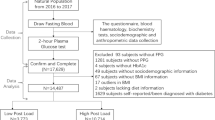
HbA1c and fasting plasma glucose (FPG) levels are commonly recognized as diagnostic indices for diabetes and glucose intolerance. However, they are not sufficient for clear detection of glucose intolerance in the early stage unless an oral glucose tolerance test (OGTT) is performed. Moreover, even in case of an OGTT, 2-h postprandial plasma glucose (PG) levels, a criterion for glucose intolerance in OGTTs, may not provide complete information regarding glucose tolerance. Whole glucose excursion after OGTT is considered to represent glucose tolerance well, and the glucose area under the curve (AUC) can be an index of glucose excursion. However, few studies have investigated measurement of the glucose AUC in glucose intolerance screening. In the present study, data from 520 OGTTs were analyzed to define the cutoff value for the glucose AUC for glucose intolerance screening. Our results showed that a cutoff value of 290 mg h/dl for the glucose AUC was highly sensitive and specific (90 and 93 %, respectively) for detecting diabetes, impaired glucose tolerance (IGT), and group at increased risk of diabetes (normal glucose tolerance with 1-h PG levels of ≥180 mg/dl after glucose load) and showed a better concordance rate than the use of HbA1c, FPG, or 2-h PG levels. Moreover, the cutoff value for the glucose AUC calculated using the diagnostic criteria in the OGTT (305 mg h/dl) was consistent with the value determined from OGTT analysis. These data suggest a possibility that glucose intolerance screening using a glucose AUC cutoff value of 290 mg h/dl could be useful.





Similar content being viewed by others
References
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention of metformin. N Engl J Med. 2002;246:393–403.
Sakane N, Sato J, Tsushita K, Tsujii S, Kotani K, Tsuzaki K, Tominaga M, Kawazu S, Sato Y, Usui T, Kamae I, Yoshida T, Kiyohara Y, Sato S, Kuzuya H, Japan Diabetes Prevention Program (JDPP) Research Group. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health. 2011;11:40–7.
Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, Nanjo K, Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, Kadowaki T, Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65–85.
Standards Australia. Australian standard glycemic index of food. Sydney: Standards Australia; 2007.
Terra SG, Somayaji V, Schwartz S, Lewin AJ, Teeter JG, Dai H, Nguyen TT, Calle RA. A dose-ranging study of the DPP-IV inhibitor PF-734200 added to metformin in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119:401–7.
Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS, Diabetes Prevention Trial-Type 1 Study Group. Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care. 2007;30:38–42.
Japan Diabetes Society. Guidance for standard treatment of diabetes mellitus 2014–2015. Bunkodo; 2014.
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Uemoto M, Murakami N, Yamakado M, Yatomi Y, Ohashi H, Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40.
Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care. 2010;33:2184–9.
Sakamoto K, Kubo F, Yoshiuchi K, Ono A, Sato T, Tomita K, Sakaguchi K, Matsuhisa M, Kaneto H, Maegawa H, Nakajima H, Kashiwagi A, Kosugi K. Usefulness of a novel system for measuring glucose area under the curve while screening for glucose intolerance in outpatients. J Diabetes Investig. 2013;4:552–9.
Sato T, Okada S, Hagino K, Asakura Y, Kikkawa Y, Kojima J, Watanabe T, Maekawa Y, Isobe K, Koike R, Nakajima H, Asano K. Measurement of glucose area under the curve using minimally invasive interstitial fluid extraction technology: evaluation of glucose monitoring concepts without blood sampling. Diabetes Technol Ther. 2011;13:1194–200.
Sakaguchi K, Hirota Y, Hashimoto N, Ogawa W, Sato T, Okada S, Hagino K, Asakura Y, Kikkawa Y, Kojima J, Maekawa Y, Nakajima H. A minimally invasive system for glucose area under the curve measurement using interstitial fluid extraction technology: evaluation of the accuracy and usefulness with oral glucose tolerance tests in subjects with and without diabetes. Diabetes Technol Ther. 2012;14:485–91.
Sakaguchi K, Hirota Y, Hashimoto N, Ogawa W, Hamaguchi T, Matsuo T, Miyagawa J, Namba M, Sato T, Okada S, Tomita K, Matsuhisa M, Kaneto H, Kosugi K, Maegawa H, Nakajima H, Kashiwagi A. Evaluation of a minimally invasive system for measuring glucose area under the curve during oral glucose tolerance tests: usefulness of sweat monitoring for precise measurement. J Diabetes Sci Technol. 2013;7:678–88.
American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37:S14–80.
International Diabetes Federation. Guideline for Management of Postmeal Glucose: Brussels; 2011.
Acknowledgments
This study was conducted by the Minimally Invasive Interstitial Fluid Extraction Technology (MIET) Study Group, which includes the following members: A. Kashiwagi, H. Maegawa (Shiga University of Medical Science), H. Kaneto (Osaka University), K. Kosugi (Osaka Police Hospital), H. Nakajima, K. Tomita (Osaka Medical Center for Cancer and Cardiovascular Diseases), M. Matsuhisa (Tokushima University), and K. Sakaguchi. This study was sponsored by Sysmex Corp.
Conflict of interest
Regarding this article, K. Sakaguchi received research funding from Sysmex Corp. H. Nakajima is a medical advisor for GlaxoSmithKline K.K. T. Sato, S. Okada, and Y. Ohnishi are employees of Sysmex Corp. No other potential conflicts of interest relevant to this article were reported.
Human rights statement and informed consent
All the data used for this study were unlinkable and anonymous. Therefore, this study is exempt from the institutional and national standards of ethics.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakaguchi, K., Takeda, K., Maeda, M. et al. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int 7, 53–58 (2016). https://doi.org/10.1007/s13340-015-0212-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-015-0212-4