Abstract
Background and Objectives
Enzalutamide is an androgen receptor inhibitor that has been approved in several countries. Absorption, distribution, metabolism, and excretion (ADME) data in animals would facilitate understanding of the efficacy and safety profiles of enzalutamide, but little information has been reported in public. The purpose of this study was to clarify the missing ADME profile in animals.
Methods
ADME of 14C-enzalutamide after oral administration as Labrasol solution were investigated in non-fasted male Sprague–Dawley rats and beagle dogs.
Results
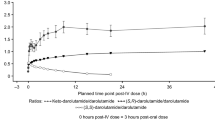
Plasma concentrations of 14C-enzalutamide peaked in rats and dogs at 6–8 h after a single oral administration. In most tissues, radioactivity concentration peaked at 4 h after administration. Excluding the gastrointestinal tract, tissues with the highest concentration of radioactivity were liver, fat, and adrenal glands. The tissue concentrations of radioactivity declined below the limit of quantitation or <0.89 % of maximum concentration by 168 h post-dose. Two known metabolites (M1 and M2) and at least 15 novel possible metabolites were detected in this study. M1 was the most abundant metabolite in both rats and dogs. Unchanged drug was a minor component in excreta. In intact rats, the mean urinary and fecal excretion of radioactivity accounted for 44.20 and 49.80 % of administered radioactivity, respectively. In intact dogs, mean urinary and fecal excretion was 62.00 and 22.30 % of the administered radioactivity, respectively.
Conclusions
Rapid oral absorption was observed in rats and dogs when 14C-enzalutamide was administered as Labrasol solution. Tissue distribution in rats was clarified. The elimination of enzalutamide is mediated primarily by metabolism. Species differences were observed in excretion route.






Similar content being viewed by others
Notes
XTANDI is a registered trademark of Astellas Pharma Inc.
References
GLOBOCAN. Estimated cancer incidence, mortality, and prevalence worldwide in 2012. 2012. http://globocan.iarc.fr/Default.aspx. Accessed 12 Aug 2016.
Lassi K, Dawson NA. Update on castrate-resistant prostate cancer: 2010. Curr Opin Oncol. 2010;22:263–7. doi:10.1097/CCO.0b013e3283380939.
Petrylak DP, Tangen CM, Hussain MHA, Lara PN, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi:10.1056/NEJMoa041318.
Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi:10.1126/science.1168175.
Scher HI, Beer TM, Higano CS, Anand A, Taplin M-E, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–46. doi:10.1016/S0140-6736(10)60172-9.
Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi:10.1056/NEJMoa1207506.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi:10.1056/NEJMoa1405095.
Gibbons JA, Ouatas T, Krauwinkel W, Ohtsu Y, van der Walt J-S, Beddo V, et al. Clinical pharmacokinetic studies of enzalutamide. Clin Pharmacokinet. 2015;54:1043–55. doi:10.1007/s40262-015-0271-5.
Ohtsu Y, Thakker DR, Gibbons JA, Tsubota K, Otsuka S, Arai H. Determination of the androgen receptor inhibitor enzalutamide and its metabolites in animal plasma and brain homogenates using LC-MS/MS and its application to pharmacokinetic studies. Chromatography. 2015;36:115–22. doi:10.15583/jpchrom.2015.029.
Penner N, Xu L, Prakash C. Radiolabeled absorption, distribution, metabolism, and excretion studies in drug development: why, when, and how? Chem Res Toxicol. 2012;25:513–31. doi:10.1021/tx300050f.
Isin EM, Elmore CS, Nilsson GN, Thompson RA, Weidolf L. Use of radiolabeled compounds in drug metabolism and pharmacokinetic studies. Chem Res Toxicol. 2012;25:532–42. doi:10.1021/tx2005212.
White RE, Evans DC, Hop CECA, Moore DJ, Prakash C, Surapaneni S, et al. Radiolabeled mass-balance excretion and metabolism studies in laboratory animals: a commentary on why they are still necessary. Xenobiotica. 2013;43:219–25. doi:10.3109/00498254.2012.706724.
Faed EM. Properties of acyl glucuronides: implications for studies of the pharmacokinetics and metabolism of acidic drugs. Drug Metab Rev. 1984;15:1213–49. doi:10.3109/03602538409033562.
ICH. Guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals M3(R2). 2009. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M3_R2/Step4/M3_R2__Guideline.pdf. Accessed 1 Sept 2016.
Bennett D, Gibbons JA, Mol R, Ohtsu Y, Williard C. Validation of a method for quantifying enzalutamide and its major metabolites in human plasma by LC-MS/MS. Bioanalysis. 2014;6:737–44. doi:10.4155/bio.13.325.
Zamek-Gliszczynski MJ, Hoffmaster KA, Nezasa K, Tallman MN, Brouwer KLR. Integration of hepatic drug transporters and phase II metabolizing enzymes: mechanisms of hepatic excretion of sulfate, glucuronide, and glutathione metabolites. Eur J Pharm Sci. 2006;27:447–86. doi:10.1016/j.ejps.2005.12.007.
Grime K, Paine SW. Species differences in biliary clearance and possible relevance of hepatic uptake and efflux transporters involvement. Drug Metab Dispos. 2013;41:372–8. doi:10.1124/dmd.112.049312.
Lai Y. Identification of interspecies difference in hepatobiliary transporters to improve extrapolation of human biliary secretion. Expert Opin Drug Metab Toxicol. 2009;5:1175–87. doi:10.1517/17425250903127234.
Varma MVS, Chang G, Lai Y, Feng B, El-Kattan AF, Litchfield J, et al. Physicochemical property space of hepatobiliary transport and computational models for predicting rat biliary excretion. Drug Metab Dispos. 2012;40:1527–37. doi:10.1124/dmd.112.044628.
Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13:519–47. doi:10.1208/s12248-011-9290-9.
Acknowledgments
We thank R. Ng of Medivation for providing enzalutamide and its metabolites, and Q. Li of Astellas Pharma and J. Mordenti of Medivation for their helpful advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Enzalutamide is co-developed by Astellas Pharma Inc and Medivation, Inc. This research was supported by co-developers of enzalutamide, as was the editorial support provided by D. Langer and A. Mason of Infusion Communications.
Conflicts of interest
Yoshiaki Ohtsu, Katsuhiro Suzuki, and Hiroshi Arai are employees of Astellas Pharma. Jacqueline A. Gibbons has declared that she receives stock options as a Medivation employee. Michael E. Fitzsimmons and Kohei Nozawa are employees of Covance and Sekisui, in which the experimental phase of this research was conducted. Other than those mentioned above, the authors report no additional conflicts of interest.
Ethical approval
All in-life portions in this report were conducted in an accredited International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) facility, which conforms to the Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Astellas Institutional Animal Care and Use Committee. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ohtsu, Y., Gibbons, J.A., Suzuki, K. et al. Absorption, Distribution, Metabolism, and Excretion of the Androgen Receptor Inhibitor Enzalutamide in Rats and Dogs. Eur J Drug Metab Pharmacokinet 42, 611–626 (2017). https://doi.org/10.1007/s13318-016-0374-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-016-0374-x