Abstract
Background and Objective
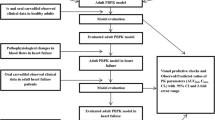
Liver cirrhosis is a complex pathophysiological condition that can affect the pharmacokinetics (PK) and hereby dosing of administered drugs. The physiologically based pharmacokinetic (PBPK) models are a valuable tool to explore PK of drugs in cirrhosis patients. The objective of this study was to develop and evaluate a PBPK-carvedilol-cirrhosis model with the available clinical data in liver cirrhosis patients and to recommend model-based drug dosing after exploring the underlying differences in unbound and total (bound and unbound) systemic carvedilol concentrations with the different disease stages.
Methods
A whole body PBPK model was developed using the population-based PBPK simulator, Simcyp®. After model development and evaluation in healthy adults, system parameters were modified according to the pathophysiological changes that occur in liver cirrhosis, and predictions were compared to available experimental data from liver cirrhosis Child–Pugh [CP]-C patients. A two-fold error range for the observed/predicted ratios (ratioObs/Pred) of the pharmacokinetic parameters was used for model evaluation. Simulations were then extended to cirrhosis CP-A and CP-B populations were no experimental data that are available to explore changes in drug disposition in these patients. Finally, drug unbound and total (bound and unbound) exposure were predicted in cirrhotic patients of different disease severity, and the results were compared to those of healthy adults.
Results
The developed model has successfully described carvedilol PK in healthy and cirrhosis CP-C patients. The model predictions showed that, there was an ~13-fold increase in unbound and ~7-fold increase in total (bound and unbound) systemic exposure of carvedilol between healthy and CP-C populations. To have comparable predicted unbound drug exposure in cirrhosis CP-A, CP-B, and CP-C populations as in healthy subjects receiving a dose of 25 mg, reductions of administered doses to 9.375 mg in CP-A, 4.68 mg in CP-B, and 2.34 mg in CP-C population were recommended.
Conclusion
The presented model-generated data can guide the optimization of carvedilol therapy on the basis of differences in unbound and total drug exposures with respect to disease severity and can help improve the design of some necessary clinical studies in the drug development process.


Presented data were extracted from Johnson et al. [7] and Simcyp® version 14 release 1. CYP cytochrome P450


The observed data are taken from the values published in literature [26]


Similar content being viewed by others
References
Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64(12):1147–61.
Morgan DJ, McLean AJ. Clinical pharmacokinetic and pharmacodynamic considerations in patients with liver disease. An update. Clin Pharmacokinet. 1995;29(5):370–91.
U.S. Food and Drug Administration Guidance for industry: pharmacokinetics in patients with impaired hepatic function. Study design, and impact on dosing and labeling. Rockville: FDA; 2003.
European Medicines Agency Committee for Medicinal Products for Human Use. Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function. London: European Medicines Agency, 2005.
Jones HM, Mayawala K, Poulin P. Dose selection based on physiologically based pharmacokinetic (PBPK) approaches. AAPS J. 2013;15(2):377–87.
Edginton AN, Willmann S. Physiology-based simulations of a pathological condition: prediction of pharmacokinetics in patients with liver cirrhosis. Clin Pharmacokinet. 2008;47(11):743–52.
Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49(3):189–206.
Li GF, Wang K, Chen R, Zhao HR, Yang J, Zheng QS. Simulation of the pharmacokinetics of bisoprolol in healthy adults and patients with impaired renal function using whole-body physiologically based pharmacokinetic modeling. Acta Pharmacol Sin. 2012;33(11):1359–71.
Rasool MF, Khalil F, Laer S. A physiologically based pharmacokinetic drug-disease model to predict carvedilol exposure in adult and paediatric heart failure patients by incorporating pathophysiological changes in hepatic and renal blood flows. Clin Pharmacokinet. 2015;54(9):943–62.
Giannelli V, Lattanzi B, Thalheimer U, Merli M. Beta-blockers in liver cirrhosis. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. 2014;27(1):20–6.
Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48(Supplement 1):S68–92.
de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53(4):762–8.
Sinagra E, Perricone G, D’Amico M, Tine F, D’Amico G. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther. 2014;39(6):557–68.
Bosch J. Carvedilol: the beta-blocker of choice for portal hypertension? Gut. 2013;62(11):1529–30.
Reiberger T, Ulbrich G, Ferlitsch A, Payer BA, Schwabl P, Pinter M, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62(11):1634–41.
Hemstreet BA. Evaluation of carvedilol for the treatment of portal hypertension. pharmacotherapy. J Hum Pharmacol Drug Ther. 2004;24(1):94–104.
Forrest EH, Bouchier IA, Hayes PC. Acute haemodynamic changes after oral carvedilol, a vasodilating beta-blocker, in patients with cirrhosis. J Hepatol. 1996;25(6):909–15.
Stanley AJ, Therapondos G, Helmy A, Hayes PC. Acute and chronic haemodynamic and renal effects of carvedilol in patients with cirrhosis. J Hepatol. 1999;30(3):479–84.
Abdelaziz A, Al-Araby M, Mahran L, Spahn-Langguth H. Active metabolites formed during hepatic first-pass: simulations featuring their contribution to the overall effect in altered liver clearance and drug-drug interactions. BMC Pharmacol. 2009;9(Suppl 2):A38.
Neugebauer G, Akpan W, Kaufmann B, Reiff K. Stereoselective disposition of carvedilol in man after intravenous and oral administration of the racemic compound. Eur J Clin Pharmacol. 1990;38(2):S108–11.
Neugebauer G, Akpan W, von Mollendorff E, Neubert P, Reiff K. Pharmacokinetics and disposition of carvedilol in humans. J Cardiovasc Pharmacol. 1987;10(Suppl 11):S85–8.
Neugebauer G, Neubert P. Metabolism of carvedilol in man. Eur J Drug Metab Pharmacokinet. 1991;16(4):257–60.
Behn F. Pharmakokinetik, Pharmakodynamik und Pharmakogenetik von Carvedilol in Abhängigkeit vom Lebensalter bei pädiatrischen Patienten mit Herzinsuffizienz. Dissertation zur Erlangung des Doktorgrades des Fachbereichs Chemie der Universität Hamburg. 2001.
Tenero D, Boike S, Boyle D, Ilson B, Fesniak HF, Brozena S, et al. Steady-state pharmacokinetics of carvedilol and its enantiomers in patients with congestive heart failure. J Clin Pharmacol. 2000;40(8):844–53.
Giessmann T, Modess C, Hecker U, Zschiesche M, Dazert P, Kunert-Keil C, et al. CYP2D6 genotype and induction of intestinal drug transporters by rifampin predict presystemic clearance of carvedilol in healthy subjects. Clin Pharmacol Ther. 2004;75(3):213–22.
Neugebauer G, Gabor M, Reiff K. Pharmacokinetics and bioavailability of carvedilol in patients with liver cirrhosis. Drugs. 1988;36(6):148–54.
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, et al. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11(2):225–37.
Berezhkovskiy LM. Volume of distribution at steady state for a linear pharmacokinetic system with peripheral elimination. J Pharm Sci. 2004;93(6):1628–40.
Wilkinson GR, Shand DG. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18(4):377–90.
Ohno A, Saito Y, Hanioka N, Jinno H, Saeki M, Ando M, et al. Involvement of human hepatic UGT1A1, UGT2B4, and UGT2B7 in the glucuronidation of carvedilol. Drug Metab Dispos Biol Fate Chem. 2004;32(2):235–9.
Takekuma Y, Yagisawa K, Sugawara M. Mutual inhibition between carvedilol enantiomers during racemate glucuronidation mediated by human liver and intestinal microsomes. Biol Pharm Bull. 2012;35(2):151–63.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9.
Meng F, Yin X, Ma X, Guo XD, Jin B, Li H. Assessment of the value of serum cholinesterase as a liver function test for cirrhotic patients. Biomed Rep. 2013;1(2):265–8.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45(9):931–56.
De Buck SS, Sinha VK, Fenu LA, Nijsen MJ, Mackie CE, Gilissen RA. Prediction of human pharmacokinetics using physiologically based modeling: a retrospective analysis of 26 clinically tested drugs. Drug Metab Dispos Biol Fate Chem. 2007;35(10):1766–80.
Khalil F, Laer S. Physiologically based pharmacokinetic models in the prediction of oral drug exposure over the entire pediatric age range-sotalol as a model drug. AAPS J. 2014;16(2):226–39.
Oldham HG, Clarke SE. In vitro identification of the human cytochrome P450 enzymes involved in the metabolism of R(+)- and S(-)-carvedilol. Drug Metab Dispos Biol Fate Chem. 1997;25(8):970–7.
Sehrt D, Meineke I, Tzvetkov M, Gultepe S, Brockmoller J. Carvedilol pharmacokinetics and pharmacodynamics in relation to CYP2D6 and ADRB pharmacogenetics. Pharmacogenomics. 2011;12(6):783–95.
Kiang TK, Ensom MH, Chang TK. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther. 2005;106(1):97–132.
Franz C, Egger S, Born C, Bravo AR, Krähenbühl S. Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis. Eur J Clin Pharmacol. 2012;68(2):179–88.
Tripathi D, Therapondos G, Lui HF, Stanley AJ, Hayes PC. Haemodynamic effects of acute and chronic administration of low-dose carvedilol, a vasodilating beta-blocker, in patients with cirrhosis and portal hypertension. Aliment Pharmacol Ther. 2002;16(3):373–80.
Hobolth L, Bendtsen F, Hansen EF, Moller S. Effects of carvedilol and propranolol on circulatory regulation and oxygenation in cirrhosis: a randomised study. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2014;46(3):251–6.
Caron G, Steyaert G, Pagliara A, Reymond F, Crivori P, Gaillard P, et al. Structure-lipophilicity relationships of neutral and protonated β-blockers, part I, intra- and intermolecular effects in isotropic solvent systems. Helv Chim Acta. 1999;82(8):1211–22.
Bachmakov I, Werner U, Endress B, Auge D, Fromm MF. Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol. 2006;20(3):273–82.
Fujimaki M, Murakoshi Y, Hakusui H. Assay and disposition of carvedilol enantiomers in humans and monkeys: evidence of stereoselective presystemic metabolism. J Pharm Sci. 1990;79(7):568–72.
von Mollendorff E, Reiff K, Neugebauer G. Pharmacokinetics and bioavailability of carvedilol, a vasodilating beta-blocker. Eur J Clin Pharmacol. 1987;33(5):511–3.
Gehr TW, Tenero DM, Boyle DA, Qian Y, Sica DA, Shusterman NH. The pharmacokinetics of carvedilol and its metabolites after single and multiple dose oral administration in patients with hypertension and renal insufficiency. Eur J Clin Pharmacol. 1999;55(4):269–77.
Acknowledgments
The authors thank Certara for providing academic research licenses for the software, Simcyp® and Phoenix WinNonLin®.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No source of funding was used in this study.
Conflict of interest
MFR, FK and SL have no potential conflicts of interest to declare.
Additional information
M. F. Rasool and F. Khalil contributed equally to this work.
Rights and permissions
About this article
Cite this article
Rasool, M.F., Khalil, F. & Läer, S. Optimizing the Clinical Use of Carvedilol in Liver Cirrhosis Using a Physiologically Based Pharmacokinetic Modeling Approach. Eur J Drug Metab Pharmacokinet 42, 383–396 (2017). https://doi.org/10.1007/s13318-016-0353-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-016-0353-2