Abstract
Background and Objective
Imatinib mesylate is presently the first-line treatment for chronic myeloid leukemia (CML). The aim of this study was to investigate the absorption and distribution kinetics of imatinib in healthy Iranian volunteers using nonlinear mixed effects modeling (NLMEM) to assess the overall, intra- and inter-subject variabilities in pharmacokinetic parameters after oral administration.
Methods
This analysis was based on data from 24 healthy subjects who participated in a bioequivalence study after administering a single dose of 200 mg of each formulation. Imatinib concentrations were quantified using a validated liquid chromatography method. To simultaneously describe the imatinib pharmacokinetic profiles obtained with both formulations, a population pharmacokinetic model was applied to data using SAEM algorithm implemented in MONOLIX, whilst simulations were used by numerical solving of ordinary differential equations to calculate secondary parameters in individuals for bioequivalence studies.
Results
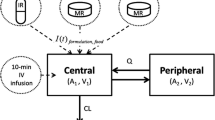
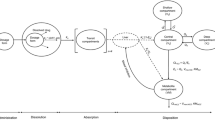
According to goodness-of-fit criteria, a two-compartment open model with sequential zero- then first-order absorption and first-order elimination was used as the structural pharmacokinetic model. Inter-individual variability (IIV) was considered for all parameters. Typical population estimates (% IIV) were fraction of the drug absorbed with a zero-order kinetic (Fr) of 0.153 (47.9 %) in period (Tk0) of 0.714 h (47.4 %), first-order absorption rate constant (k a) of 0.94 h−1(31.2 %), oral clearance of 19 L/h (27.9 %), central volume of distribution (V c/F) of 139 L (21.5 %), apparent peripheral volume of distribution (V p/F) of 130 L (29.7 %) and the apparent inter-compartment clearance (Q/F) of 29.6 L/h (41.8 %). Body mass index (BMI) was the only covariate found to significantly affect V p /F. The coefficient of variation for intra-individual plasma exposure (AUC0–∞) was 27.8 %.
Conclusions
Analyses using NLMEM for imatinib exhibited absorption complexities such as two input rates and medium to high intra-individual variability in drug exposure.





Similar content being viewed by others
References
Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, Druker BJ, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56(1):100–4.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR–ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80.
Golabchifar AA, Rezaee S, Ghavamzadeh A, Alimoghaddam K, Dinan NM, Rouini MR. Population pharmacokinetics of imatinib in Iranian patients with chronic-phase chronic myeloid leukemia. Cancer Chemother Pharmacol. 2014;74(1):85–93.
Peng B, Dutreix C, Mehring G, Hayes MJ, Ben-Am M, Seiberling M, et al. Absolute bioavailability of imatinib (Glivec) orally versus intravenous infusion. J Clin Pharmacol. 2004;44(2):158–62.
Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44(9):879–94.
Eechoute K, Sparreboom A, Burger H, Franke RM, Schiavon G, Verweij J, et al. Drug transporters and imatinib treatment: implications for clinical practice. Clin Cancer Res. 2010;17(3):406–15.
Haouala A, Widmer N, Duchosal MA, Montemurro M, Buclin T, Decosterd LA. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood. 2011;117(8):75–87.
Burger H, Nooter K. Pharmacokinetic resistance to imatinib mesylate: role of the ABC drug pumps ABCG2 (BCRP) and ABCB1 (MDR1) in the oral bioavailability of imatinib. Cell Cycle. 2004;3(12):1502–5.
FDA. Bioavailability and bioequivalence studies for orally administered drug products - general considerations. Guidance for Industry, Revision 1; 2003.
EMA. Guideline on the investigation of bioequivalence. CPMP/EWP/QWP/1401/98 Rev. 1/Corr**; 2010.
Jusko WJ, Koup JR, Alvan G. Nonlinear assessment of phenytoin bioavailability. J Pharmacokinet Biopharm. 1976;4(4):327–36.
Hayashi N, Aso H, Higashida M, Kinoshita H, Ohdo S, Yukawa E, et al. Estimation of rhG-CSF absorption kinetics after subcutaneous administration using a modified Wagner–Nelson method with a nonlinear elimination model. Eur J Pharm Sci. 2001;13(2):151–8.
Dubois A, Gsteiger S, Pigeolet E, Mentre F. Bioequivalence tests based on individual estimates using non-compartmental or model-based analyses: evaluation of estimates of sample means and type I error for different designs. Pharm Res. 2010;27(1):92–104.
Dubois A, Lavielle M, Gsteiger S, Pigeolet E, Mentre F. Model-based analyses of bioequivalence crossover trials using the stochastic approximation expectation maximisation algorithm. Stat Med. 2011;30(21):2582–600.
Dubois A, Gsteiger S, Balser S, Pigeolet E, Steimer JL, Pillai G, et al. Pharmacokinetic similarity of biologics: analysis using nonlinear mixed-effects modeling. Clin Pharmacol Ther. 2012;91(2):234–42.
Delbaldo C, Chatelut E, Re M, Deroussent A, Seronie-Vivien S, Jambu A, et al. Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res. 2006;12(20 Pt 1):6073–8.
Judson I, Ma P, Peng B, Verweij J, Racine A, di Paola ED, et al. Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol. 2005;55(4):379–86.
Menon-Andersen D, Mondick JT, Jayaraman B, Thompson PA, Blaney SM, Bernstein M, et al. Population pharmacokinetics of imatinib mesylate and its metabolite in children and young adults. Cancer Chemother Pharmacol. 2009;63(2):229–38.
Schmidli H, Peng B, Riviere GJ, Capdeville R, Hensley M, Gathmann I, et al. Population pharmacokinetics of imatinib mesylate in patients with chronic-phase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol. 2005;60(1):35–44.
Petain A, Kattygnarath D, Azard J, Chatelut E, Delbaldo C, Geoerger B, et al. Population pharmacokinetics and pharmacogenetics of imatinib in children and adults. Clin Cancer Res. 2008;14(21):7102–9.
Widmer N, Decosterd LA, Csajka C, Leyvraz S, Duchosal MA, Rosselet A, et al. Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br J Clin Pharmacol. 2006;62(1):97–112.
Golabchifar AA, Rouini MR, Shafaghi B, Rezaee S, Foroumadi A, Khoshayand MR. Optimization of the simultaneous determination of imatinib and its major metabolite, CGP74588, in human plasma by a rapid HPLC method using D-optimal experimental design. Talanta. 2011;85(5):2320–9.
Panhard X, Samson A. Extension of the SAEM algorithm for nonlinear mixed models with 2 levels of random effects. Biostatistics. 2009;10(1):121–35.
Ette EI, Sun H, Ludden TM. Balanced designs in longitudinal population pharmacokinetic studies. J Clin Pharmacol. 1998;38(5):417–23.
Karlsson M, Holford N. A tutorial on visual predictive checks, PAGE 17 Abstr 1434. 2008. Available at www.page-meeting.org/?abstract=1434. Accessed 17 July 2015.
Lavielle M, Mesa H. Improved diagnostic plots require improved statistical tools. Implementation in MONOLIX 4.0, PAGE 20 Abstr 2180. 2011. Available at www.page-meeting.org/?abstract=2180. Accessed 17 July 2015.
Baek IH, Lee BY, Kang W, Kwon KI. Comparison of average, scaled average, and population bioequivalence methods for assessment of highly variable drugs: an experience with doxifluridine in beagle dogs. Eur J Pharm Sci. 2010;39(1–3):175–80.
Hellriegel ET, Bjornsson TD, Hauck WW. Interpatient variability in bioavailability is related to the extent of absorption: implications for bioavailability and bioequivalence studies. Clin Pharmacol Ther. 1996;60(6):601–7.
Holford NH, Ambros RJ, Stoeckel K. Models for describing absorption rate and estimating extent of bioavailability: application to cefetamet pivoxil. J Pharmacokinet Biopharm. 1992;20(5):421–42.
Yoo C, Ryu MH, Kang BW, Yoon SK, Ryoo BY, Chang HM, et al. Cross-sectional study of imatinib plasma trough levels in patients with advanced gastrointestinal stromal tumors: impact of gastrointestinal resection on exposure to imatinib. J Clin Oncol. 2010;28(9):1554–9.
Egorin MJ, Shah DD, Christner SM, Yerk MA, Komazec KA, Appleman LR, et al. Effect of a proton pump inhibitor on the pharmacokinetics of imatinib. Br J Clin Pharmacol. 2009;68(3):370–4.
Sparano BA, Egorin MJ, Parise RA, Walters J, Komazec KA, Redner RL, et al. Effect of antacid on imatinib absorption. Cancer Chemother Pharmacol. 2009;63(3):525–8.
Illmer T, Schaich M, Platzbecker U, Freiberg-Richter J, Oelschlagel U, von Bonin M, et al. P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia. 2004;18(3):401–8.
Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104(12):3739–45.
Angelini S, Soverini S, Ravegnini G, Barnett M, Turrini E, Thornquist M, et al. Association between imatinib transporters and metabolizing enzymes genotype and response in newly diagnosed chronic myeloid leukemia patients receiving imatinib therapy. Haematologica. 2013;98(2):193–200.
Giannoudis A, Wang L, Jorgensen AL, Xinarianos G, Davies A, Pushpakom S, et al. The hOCT1 SNPs M420del and M408 V alter imatinib uptake and M420del modifies clinical outcome in imatinib-treated chronic myeloid leukemia. Blood. 2013;121(4):628–37.
Di Paolo A, Polillo M, Capecchi M, Cervetti G, Baratà C, Angelini S, et al. The c.480C>G polymorphism of hOCT1 influences imatinib clearance in patients affected by chronic myeloid leukemia. Pharmacogenomics J. 2014;14(4):328–35.
Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, et al. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64(7):2333–7.
van Erp NP, Gelderblom H, Karlsson MO, Li J, Zhao M, Ouwerkerk J, et al. Influence of CYP3A4 inhibition on the steady-state pharmacokinetics of imatinib. Clin Cancer Res. 2007;13(24):7394–400.
Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–47.
Chowdhury MM, Kim DH, Ahn JK. A physiologically based pharmacokinetic model for absorption and distribution of imatinib in human body. Bull Korean Chem Soc. 2011;32(11):3967–72.
Acknowledgments
This study was part of a PhD thesis supported by Tehran University of Medical Sciences (TUMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was financially supported by Osvah Pharmaceutical Co., Iran.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures in this study were in accordance with the 1964 Helsinki declaration. The study was approved by ethics committee of Tehran University of Medical Sciences.
Informed consent
All subjects gave written informed consent and details of study were explained to all volunteers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Golabchifar, AA., Rezaee, S., Dinan, N.M. et al. Population Pharmacokinetic Analysis of the Oral Absorption Process and Explaining Intra-Subject Variability in Plasma Exposures of Imatinib in Healthy Volunteers. Eur J Drug Metab Pharmacokinet 41, 527–539 (2016). https://doi.org/10.1007/s13318-015-0292-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-015-0292-3