Abstract
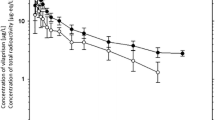
Nomegestrol acetate (NOMAC), a synthetic progestogen derived from 19-norprogesterone, is an orally active drug with a strong affinity for the progesterone receptor. NOMAC inhibits ovulation and is devoid of undesirable androgenic and estrogenic activities. The aim of this study was to evaluate the pharmacokinetics, tissue distribution, and excretion of NOMAC in female rats. Sprague–Dawley female rats were orally administered a single dose of NOMAC (10, 20 or 40 mg/kg) and drug plasma concentrations at different times were determined by RP-HPLC. Tissue distribution at 1, 2, and 4 h and excretion of NOMAC into bile, urine, and feces after dosing were investigated. The results showed that NOMAC was rapidly absorbed after oral administration, with \(t_{ \hbox{max} }\) of 1–2 h. The plasma concentration–time curves were fitted in a two-compartment model. The exposure to NOMAC (\(C_{ \hbox{max} }\) and \({\text{AUC}}\)) increased dose proportionally from 10 to 40 mg/kg. The average CL and \(t_{1/2\beta }\) were 5.58 L/(h·kg) and 10.8 h, respectively. The highest concentrations of NOMAC in ovary, liver, kidney, lung, heart, brain, spleen, muscle, and uterus were observed at 2 h, whereas the highest concentrations in stomach, pituitary, and hypothalamus appeared at 1 h. The total cumulative excretion of NOMAC in feces (0–72 h), urine (0–72 h), and bile (0–48 h) was ~1.06, 0.03, and 0.08 % of the oral administered dose, respectively. This study indicated that NOMAC had a widespread distribution in tissues, including ovary, pituitary, and hypothalamus, which are main target tissues where NOMAC inhibits ovulation. NOMAC was excreted via both feces and urine with few unchanged NOMAC excreted. Enterohepatic circulation was found in the drug elimination; however, it did not significantly affect \(t_{ \hbox{max} }\).





Similar content being viewed by others
Abbreviations
- NOMAC:
-
Nomegestrol acetate
- HPLC:
-
High-pressure liquid chromatography
- AUC:
-
Area under the plasma concentration–time curve
- CL:
-
Clearance
- \(C_{ \hbox{max} }\) :
-
Maximum plasma concentration
- V/F :
-
Apparent distribution volume
- \(t_{1/2\beta }\) :
-
Terminal half-life
- \(t_{ \hbox{max} }\) :
-
Time to maximum plasma concentration
- K a :
-
Absorption rate constant
- K 10 :
-
Elimination rate constant
- K 12 :
-
Distribution rate constant from the central compartment to the peripheral compartment
- K 21 :
-
Distribution rate constant from the peripheral compartment to the central compartment
- α :
-
Rate constant associated with the distribution phase of the concentration–time curve
- β :
-
Rate constant associated with the terminal phase of the concentration–time curve
References
Alsina JC (2010) After 50 years of ethinylestradiol, another oestrogen in combined oral contraceptives. Eur J Contracept Reprod Health Care 15:1–3
Bazin B, Thevenot R, Bursaux C, Paris J (1987) Effect of nomegestrol acetate, a new 19-nor-progesterone derivative, on pituitary-ovarian function in women. Br J Obstet Gynaecol 94:1199–1204
Botella J, Porthe-Nibelle J, Paris J, Lahlou B (1986) Interaction of new 19-nor progesterone derivatives with progestagen, mineralocorticoid and glucocorticoid cytosolic receptors. J Pharmacol 17:699–706
Botella J, Paris J, Duc I, Lahlou B (1988) Nomegestrol acetate binding to cytosolic progesterone receptor in human endometrium. Med Sci Res 16:299–300
Calabrese EJ (1983) Principles of Animal Extrapolation. Wiley, New York
Couzinet B, Young J, Kujas M, Meduri G, Brailly S, Thomas JL, Chanson P, Schaison G (1999) Theantigonadotropic activity of a 19-nor-progesterone derivative is exerted both at the hypothalamic and pituitary levels in women. J Clin Endocrinol Metab 84:4191–4196
Duc I, Botella J, Gillet JY, Duforestel T, Paris J (1990) Nomegestrol acetate binding to human breast tissue. Med Sci Res 18:57–58
Ezan E, Benech H, Bucourt R, Ardouin T, Tchernatinsky C, Thomas JL, Paris J, Grognet JM (1993) Enzyme immunoassay for nomegestrol acetate in human plasma. J Steroid Biochem Mol Biol 46:507–514
Gerrits MG, Schnabel PG, Post TM, Peeters PA (2013) Pharmacokinetic profile of nomegestrol acetate and 17β-estradiol after multiple and single dosing in healthy women. Contraception 87:193–200
Gibaldi M, Perrier D (1982) Pharmacokinetics. Marcel Dekker, New York
Huang Q, Cao L, Gu Z (2000) RP-HPLC determination of nomegestrol acetate in plasma of rabbit. Chin J Pharm Anal 20:379–380
Huang Q, Chen X, Zhu Y, Cao L (2014) Development and validation of an HPLC method for the quantitation of nomegestrol acetate in biological matrices of rats. Biomed Chromatogr (Submitted)
Jamin C (1992) Female contraception by a normal dose progestogen after 40 years of age. Possible association of nomegestrol-17-beta-estradiol acetate by percutaneous route. Rev Fr Gynecol Obstet 87:370–376
Lello S (2010) Nomegestrol acetate: pharmacology, safety profile and therapeutic efficacy. Drugs 70:541–559
Merk Sharp and Dohme (Australia) Pty Limited (2011) Australian public assessment report for nomegestrol acetate/oestradiol. Health safety regulation http://www.tga.gov.au/pdf/auspar/auspar-zoely.pdf. Accessed 13 April 2014
Miyake T, Rooks WHII (1966) The relation between the structure and physiological activity of progestational steroids. In: Dorfman RI (ed) Methods in hormone research, 5th edn. Academic, New York, pp 59–145
Ruan X, Seeger H, Mueck AO (2012) The pharmacology of nomegestrol acetate. Maturitas 71:345–353
Shields-Botella J, Duc I, Duranti E, Puccio F, Bonnet P, Delansorne R, Paris J (2003) An overview of nomegestrol acetate selective receptor binding and lack of estrogenic action on hormone-dependent cancer cells. J Steroid Biochem Mol Biol 87:111–122
Thevenot R (1984) Nomegestrol acetate. Drugs Future 9:657–659
van Diepen HA (2012) Preclinical pharmacological profile of nomegestrol acetate, a synthetic 19-nor-progesterone derivative. Reprod Biol Endocrinol 8:10–85
Yang LP, Plosker GL (2012) Nomegestrol acetate/estradiol: in oral contraception. Drugs 72:1917–1928
Yi Z (1992) PK-GRAPH software to calculate pharmacokinetic parameters. Acta Univ Med Second Shanghai 12:272–274
Acknowledgments
The authors would like to thank Gengdi You and Rongfa Lu for excellent technical support. This work was financially supported by Shanghai Modern Biology and Drug Industry Development Foundation (No. 955419004).
Conflict of interest
The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Q. Huang and X. Chen contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, Q., Chen, X., Zhu, Y. et al. Pharmacokinetics, tissue distribution, and excretion of nomegestrol acetate in female rats. Eur J Drug Metab Pharmacokinet 40, 435–442 (2015). https://doi.org/10.1007/s13318-014-0224-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-014-0224-7