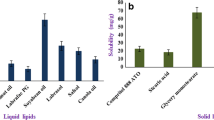
Abstract
Progress in nanoscience and nanotechnology laid foundation for nanotherapy-based approach in cancer drug delivery for improved therapy and quality of life. The prepared polymeric nanoparticles (PNPs), PCL, PLGA and PLA NPs help in delivering paclitaxel (TAX) in vivo by avoiding the use of unsafe excipient, Cremophore EL. The classy microscopic examination SEM, TEM and AFM analysis revealed the spherical and smooth structure of the NPs as well as their homogeneous solid matrix without any amorphous arrangements. The FTIR analysis of PNPs exposed that there was no chemical interaction between polymer, stabilizer and TAX. The 1H NMR and XRD analyses illustrate molecular dispersion of TAX in the polymeric matrix and no evidence was observed for the presence of crystalline TAX. The outcome of in vivo acute toxicity study endorses residual solvent free PNPs. The PNPs demonstrate excellent control in delivering TAX up to 48 h with best fitted to First-order, Baker-Lonsdale, Higuchi and Korsmeyer-Peppas model. The log plasma concentration–time profile shows that the prepared PNPs were safe and have much less side-effects. The pharmacokinetic study results illustrate increase in mean residence time as result of long circulating nature of the prepared nanoparticles, which helps them to reach target area. The estimated pharmacokinetic parameters AUC0–∞ (ng h)/mL, AUMC0–∞ (ng h2)/mL, C max (ng/mL), t 1/2 (h), MRT (h), Cl (L/h/kg), V ss (L/kg) and V z (L/kg) shows improved therapeutic efficacy when compared with TAX solution.










Similar content being viewed by others
References
Allwood MC, Martin H (1996) The extraction of diethylhexylphthalate (DEHP) from polyvinyl chloride components of intravenous infusion containers and administration sets by paclitaxel injection. Int J Pharm 127:65–71
Brittain HG (2009) Profiles of drug substances, excipients, and related methodology. In: Jauhari S, Singh S, Dash AK (ed) Paclitaxel. Academic Press, Elsevier, London, pp. 299–344
Chakravarthi SS, De S, Miller DW, Robinson DH (2010) Comparison of anti-tumor efficacy of paclitaxel delivery in nano- and microparticles. Int J Pharm 383:37–44
Champion JA, Katare YK, Mitragotri S (2007) Particle shape: a new design parameters for micro and nanoscale drug delivery carriers. J Control Release 121:3–9
Derakhshandeh K, Erfan M, Dadashzadeh S (2007) Encapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: factorial design, characterization and release kinetics. Eur J Pharm Biopharm 66:34–41
Dhanikula B, Singh DR, Panchagnula R (2005) In vivo pharmacokinetic and tissue distribution studies in mice of alternative formulations for local and systemic delivery of paclitaxel: gel, film, prodrug, liposomes and micelles. Curr Drug Deliv 2:35–44
Dong Y, Feng S (2007) In vitro and in vivo evaluation of methoxy polyethylene glycol-polylactide (MPEG-PLA) nanoparticles for small-molecular drug chemotherapy. Biomaterials 28:4154–4160
Elzein T, Awada H, Eddine MN, Delaite C, Brogly M (2005) A model of chain folding in polycaprolactone-b-polymethyl methacrylate diblock copolymer. Thin Solid Films 483:388–395
Gagandeep S, Novikoff PM, Ott M, Gupta S (1999) Paclitaxel shows cytotoxic activity in human hepatocellular carcinoma cell lines. Cancer Lett 136:109–118
Gill KK, Nazzal S, Kaddoumi A (2011) Paclitaxel loaded PEG5000-DSPE micelles as pulmonary delivery platform: formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluation. Eur J Pharm Biopharm 79:276–284
Jin C, Li H, He Y, He M, Bai L, Cao Y, Song W, Dou K (2010) Combination chemotherapy of doxorubicin and paclitaxel for hepatocellular carcinoma in vitro and in vivo. J Cancer Res Clin Oncol 136:267–274
Lao LL, Venkataraman SS, Peppas NA (2008) Modeling of drug release from biodegradable polymer blends. Eur J Pharm Biopharm 70:796–803
Mainardes RM, Evangelista RC (2005) PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. Int J Pharm 5290:137–144
Mitra A, Lin S, Pharm J (2003) Effect of surfactant on fabrication and characterization of paclitaxel-loaded polybutylcyanoacrylate nanoparticulate delivery systems. Pharmacology 55:895–902
Owens ED, Peppas NA (2006) Opsonization, biodistribution and pharmacokinetics of polymer nanoparticles. Int J Pharm 307:93–102
Panyam J, Williams D, Dash A, Leslie-Pelecky D, Labhasetwar V (2004) Solid-state solubility influences encapsulation and release of hydrophobic drugs from PLGA/PLA nanoparticles. J Pharm Sci 93:1804–1814
Saha RN, Vasanthakumar S, Benda G, Snehalatha M (2010) Nanoparticulate drug delivery systems for chemotherapy. Mol Membr Biol 27:215–223
Sahana DK, Mittal G, Bhardwaj V, Ravikumar MNV (2008) PLGA nanoparticles for oral delivery of hydrophobic drugs: influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. J Pharm Sci 97:1530–1542
Sahoo SK, Panyam J, Prabha S, Labhasetwar V (2002) Residual polyvinyl alcohol associated with poly (lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release 82:105–114
Seremeta KP, Chiappetta DA, Sosnik A (2013) Poly (caprolactone), Eudragit® RS 100 and Poly (caprolactone)/Eudragit® RS 100 blend submicron particles for the sustained release of the antiretroviral efavirenz. Colloids Surf Biointerface 102:441–449
Serrano ABG, Martin MA, Bravo L, Goya L, Ramos S (2009) A diet rich in cocoa attenuates N-nitrosodiethylamine-induced liver injury in rats. Toxicology 47:2499–2506
Shah N, Chaudhari K, Dantuluri P, Murthy RSR, Das S (2009) Paclitaxel-loaded PLGA nanoparticles surface modified with transferring and Pluronic P85, an in vitro cell line and in vivo biodistribution studies on rat model. J Drug Target 17:533–542
Sinha B, Mukherjee B, Pattnaik G (2013) Poly lactide-co-glycolide nanoparticles containing variconazole for pulmonary delivery: in vitro and in vivo study. Nanomed Nanotechnol Biol Med 9:94–104
Sinjan De, Miller DW, Robinson DH (2005) Effect of particle size of nanospheres and microspheres on the cellular-association and cytotoxicity of paclitaxel in 4T1 cells. Pharm Res 22:766–775
Sivacharan K, Girish B, Snehalatha M, Saha RN (2010) Application of rotatable central composite design in the preparation and optimization of poly (lactic-co-glycolic acid) nanoparticles for controlled delivery of paclitaxel. Drug Dev Ind Pharm 36:1377–1387
Snehalatha M, Venugopal K, Saha RN, Babbar AK, Sharma RK (2008a) EP loaded PLGA and PCL nanoparticles II: biodistribution and pharmacokinetic after radiolabeling with Tc-99m. Drug Deliv 15:277–287
Snehalatha M, Venugopal K, Saha RN (2008b) Etoposide-loaded PLGA and PCL nanoparticles I: preparation and effect of formulation variables. Drug Deliv 15:267–275
Straub JA, Chickering DE, Lovely JC, Zhang H, Shah B, Waud WR, Bernstein H (2005) Intravenous hydrophobic drug delivery: a porous particle formulation of paclitaxel (AI-850). Pharm Res 22:347–355
Vasanthakumar S, Srinath ST, Saha RN (2012) A simple and rapid 3D view method for selective and sensitive determination of paclitaxel in micro volume rat plasma by LC-diode array UV and its pharmacokinetic application. J Chromatogr Sci 50:259–270
Vasanthakumar S, Karthikeyan V, Rashid A, Saha RN (2013) Determination of paclitaxel by 3D view LC-diode array UV: its application to an in situ closed loop re-circulating intestine absorption study in rats. J Liq Chromatogr Related Technol 36(19):2698–2730
Verger ML, Fluckiger L, Kim YI, Hoffman M, Maincent P (1998) Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur J Pharm Biopharm 46:137–143
Yang T, Cui F, Choi M, Cho J, Chung S, Shim C, Kim D (2007) Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm 338:317–326
Yeh TK, Ze Lu, Wientjes MG, Au JLS (2005) Formulating paclitaxel in nanoparticles alters its disposition. Pharm Res 22:867–874
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, Xie S (2010a) An add-in program for modeling and comparison of drug dissolution profiles. AAPS J 12:263–271
Zhang Y, Tang L, Sun L, Bao J, Song C, Huang L, Liu K, Tian Y, Tian Ge, Li Z, Sun H, Mei L (2010b) A novel paclitaxel-loaded poly (ε-caprolactone)/poloxamer 188 blend nanoparticles overcoming multidrug resistance for cancer treatment. Acta Biomater 6:2045–2052
Acknowledgments
One of the authors, Sekar VasanthaKumar (SVK) acknowledges Council of Scientific and Industrial Research (CSIR, New Delhi, India) for providing scholarship, Senior Research Fellow (SRF). Authors are grateful to Getwell Pharmaceuticals (New Delhi, India) for generous gift samples of TAX. Authors appreciate the services provided by Central Electronics Engineering Research Institute (Rajasthan, India) and Sophisticated Analytical Instrument Facility, Indian Institute of Technology (Mumbai, India) to analyze our samples by Scanning Electron Microscopy, Atomic Force Microscopy and Transmission Electron Microscopy and Nuclear Magnetic Resonances. Last but not least, author SVK appreciates Dr. P SenthilKumar, Assistant Professor, KMCH College of Pharmacy, Coimbatore, Tamil Nadu, India for his help during literature review.
Author contribution
Sekar VasanthaKumar made substantial contribution to conception and design of the entire research work. He was involved in conducting all the experiments and obtaining data. He contributed significantly in analyzing and interpreting the obtained data. He was involved in making the draft manuscript and critically reviewing the draft. Ranendra N. Saha equally made substantial contribution to conception and design of the entire research work. He critically reviewed the draft manuscript and gave final approval of the version to be submitted. Haja Nazeer Ahamed helped in conception and design of in vivo acute toxicity study. He also contributed in analyzing and interpreting of the toxicity study result.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
VasanthaKumar, S., Ahamed, H.N. & Saha, R.N. Nanomedicine I: In vitro and in vivo evaluation of paclitaxel loaded poly-(ε-caprolactone), poly (dl-lactide-co-glycolide) and poly (dl-lactic acid) matrix nanoparticles in wistar rats. Eur J Drug Metab Pharmacokinet 40, 137–161 (2015). https://doi.org/10.1007/s13318-014-0189-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-014-0189-6