Abstract
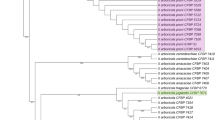
Ray blight is a destructive disease of Asteraceae affecting chrysanthemum and pyrethrum industries worldwide. Three morphologically similar but phylogenetically distinct species of the family Didymellaceae; Stagonosporopsis chrysanthemi, S. inoxydabilis and S. tanaceti are associated with the disease. Despite their close evolutionary relationship and cross host pathogenicity, these species have marked differences in their biology and epidemiology. Stagonosporopsis chrysanthemi and S. inoxydabilis are both homothallic with a MAT locus that carries both mating type genes. Ascomata play a major role in survival and dispersal of S. chrysanthemi, contributing to the onset of ray blight epidemics on chrysanthemum. However, S. tanaceti is either asexual or heterothallic due to the presence of only MAT1-2 idiomorph in its genome. Morphological similarity of the species causing ray blight on various Asteraceae, multiple changes in their taxonomy and historical confusion with other Phoma-like species have resulted in lack of clarity in their taxonomy, host range and global distribution. Host specificity studies and reports on global distribution of S. chrysanthemi published before its separation from S. inoxydabilis should be treated with caution. A recently developed species-specific multiplex PCR assay provides a rapid and robust tool to study the global distribution of these important quarantine pathogens. When global populations from cultivated and/or wild Asteraceae are available, population genetic analyses may aid in understanding the origin, evolutionary history and global migration patterns of the Stagonosporopsis spp. associated with ray blight of Asteraceae.


Similar content being viewed by others
References
Albinati A, Arnone A, Assante G, Meille SV, Nasini G (1989) Chrysanthone, a bioactive alkaloid from Ascochyta chrysanthemi. Phytochemistry 28:923–927
Anonymous (1955) Plant disease survey for the twelve months ending 30th June 1955. Twenty–fifth annual report N.S.W. Department of Agriculture. Biological Branch–Division of Science Services, New South Wales, Australia
Arnone A, Assante G, Nasin G, de Pava OV (1990) Chrysanthones B and C, secondary metabolites produced by the fungus Ascochyta chrysanthemi. Phytochemistry 29:2499–2502
Arzanlou M, Crous PW, Zwiers LH (2010) Evolutionary dynamics of mating-type loci of Mycosphaerella spp. occurring on banana. Eukaryot Cell 9:164–72
Aveskamp M, De Gruyter J, Woudenberg J, Verkley G, Crous PW (2010) Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related Pleosporalean genera. Stud Mycol 65:1–60
Baker KF, Dimock AW, Davis LH (1949) Life history and control of the Ascochyta ray blight of chrysanthemum. Phytopathol 39:789–805
Baker KF, Dimock AW, Davis LH (1961) Cause and prevention of the rapid spread of the Ascochyta disease of chrysanthemum. Phytopathol 51:96–101
Barrett LG, Thrall PH, Burdon JJ, Linde CC (2008) Life history determines genetic structure and evolutionary potential of host-parasite interactions. Trends Ecol Evol 23:678–685
Bhat B, Menary R (1984) Pyrethrum production in Australia: its past and present potential. J Aust I Agr Sci 50:189–92
Bhuiyan MAHB, Groom T, Nicolas ME, Taylor PWJ (2015) Histopathology of Stanaceti infection in pyrethrum leaf lamina. Australas Plant Pathol 44:629–636
Bhuiyan MAHB, Groom T, Nicolas ME, Taylor PWJ (2016) Infection process of Stagonosporopsis tanaceti in pyrethrum seed and seedlings. Plant Pathol
Blakeman JP, Dickinson CH (1967) The effect of ultraviolet and visible light on infection of host leaf tissue by four species of Ascochyta. Trans Br Mycol Soc 50:385–396
Blakeman J, Hadley G (1968) The pattern of asexual sporulation in Mycosphaerella ligulicola. Trans Br Mycol Soc 51:643–51
Blakeman J, Hornby D (1966) The persistence of Colletotrichum coccodes and Mycosphaerella ligulicola in soil, with special reference to sclerotia and conidia. Trans Br Mycol Soc 49:227–40
Boerema G, Bollen G (1975) Conidiogenesis and conidial septation as differentiating criteria between Phoma and Ascochyta. Persoonia 8:111–4
Boerema GH, Verhoeven AA (1979) Check-list for scientific names of common parasitic fungi. Series 2c: fungi on field crops: pulse (legumes), and forage crops (herbage legumes). Neth J Plant Pathol 85:151–185
Boerema GH, De Gruyter J, Noordeloos ME (1997) Contributions towards a monograph of Phoma (Coelomycetes)–IV Section Heterospora: taxa with large sized conidial dimorphs, in vivo sometimes as Stagonosporopsis synanamorphs. Persoonia 16:335–371
Boerema GH, De Gruyter J, Noordeloos M, Hamers M (2004) Phoma identification manual. Differentiation of specific and infra–specific taxa in culture. Wallingford CABI
Chesters CGC, Blakeman JP (1966) The survival on chrysanthemum roots of epiphytic mycelium of Mycosphaerella ligulicola. Ann Appl Biol 58:291–8
Chesters CGC, Blakeman JP (1967) Host range and variation in virulence of Mycosphaerella ligulicola. Ann Appl Biol 60:385–390
Chilvers MI, Jones S, Meleca J, Peever TL, Pethybridge SJ, Hay FS (2014) Characterization of mating type genes supports the hypothesis that Stagonosporopsis chrysanthemi is homothallic and provides evidence that Stagonosporopsis tanaceti is heterothallic. Curr Genet 60:295–302
Clements FE, Shear CL (1931) The genera of fungi. Wilson, New York
Crane GL, Jackson C, Myers F (1970) Fungicidal control of Ascochyta chrysanthemi. P Fl St Hortic Soc 82:379–380
Da Silva JAT (2003) Chrysanthemum: advances in tissue culture, cryopreservation, postharvest technology, genetics and transgenic biotechnology. Biotechnol Adv 21:715–766
De Gruyter J, Boerema GH, Van der Aa HA (2002) Contributions towards a monograph of Phoma (Coelomycetes). VI-2. Section Phyllostictoides: outline of its taxa. Persoonia 18:1–53
Diedicke H (1912) Die Abteilung Hyalodidymae der Sphaerioideen. Ann Mycol 10:135–152
EFSA PLH Panel (European Food Safety Authority Panel on Plant Health) (2013) Scientific opinion on the risks to plant health posed by Stagonosporopsis chrysanthemi (Stevens) Crous, Vaghefi and Taylor [Didymella ligulicola (Baker, Dimock and Davis) Arx var. ligulicola; syn. Didymella ligulicola (Baker, Dimock and Davis) Arx] in the EU territory, with identification and evaluation of risk reduction options. EFSA J 11:3376
Engelhard AW (1984) New fungicides for control of Ascochyta blight of chrysanthemum. P Fl St Hortic Soc 97:292–294
EPPO (1980) Data sheets on quarantine organisms List A2. Didymella chrysanthemi (Tassi) Garibaldi & Gullino. Paris. rue Le Notre
EPPO (1982) Datasheets on quarantine organisms Set 5 List A2 (quarantine organisms present in some EPPO countries). EPPO Bulletin 12
EPPO (2016) PQR–EPPO database on quarantine pests (available online). http://www.eppo.int Accessed 26 June 2016
Fides BV (2006) Mum-manual for all-year-round chrysanthemums. De Lier, Netherlands, 40 pp
Fox R (1998) Chrysanthemum ray blight. Mycologist 12:35–6
Fujioka Y (1952) List of Crop Diseases in Japan. Econ. Sci. Sect., Nat. Resources Div., Gen. Headquart., Supr. Command, Allied Powers, Tokyo. Prelim Study 73:1–212
Gambogi P (1963) Mycosphaerella ligulicola Baker et al., f.c, Ascochyta chrysanthemi Stevens, la micosi ligulare del cr isantemo. L’Agricoltura ltaliana 63:87–92
Garibaldi A, Gullino G (1971) Brevi notizie sulla presenza in Italia dell’ascochitosi del crisantemo. L’Agricoltura Italiana 71:21–290
Green JL, Engelhard AW (1974) An integrated production system to increase chrysanthemum yield and ascochyta blight control. Florida State Horticultural Society, pp. 496–500
Hay FS, Gent DH, Pilkington SJ, Pearce TL, Scott JB, Pethybridge SJ (2015) Changes in distribution and frequency of fungi associated with a foliar disease complex of pyrethrum in Australia. Plant Dis 99:1227–1235
Horst RK, Nelson PE (1997) Compendium of chrysanthemum diseases. American Phytopathological Society, APS Press
Jones S (2009) Characterisation of cultural, biological and molecular variability of Phoma ligulicola isolates associated with ray blight disease of pyrethrum and chrysanthemum. Dissertation, University of Tasmania, Australia
Jones S, Pethybridge S, Hay F, Groom T, Wilson C (2007) Baseline sensitivity of Australian Phoma ligulicola isolates from pyrethrum to azoxystrobin and difenoconazole. J Phytopathol 155:377–80
Kim DK, Shim CK, Bae DW, Lee SC, Kim HK (2001) Occurrence of blossom blight of Chrysanthemum boreale caused by Didymella chrysanthemi. Plant Pathol J 17:347–349
McCoy RE (1973) Ballistics of Mycosphaerella ligulicola ascospore discharge. Phytopathol 63:793–794
McCoy RE, Dimock AW (1970) Response of chrysanthemum to infection by Mycosphaerella ligulicola. Phytopathol 60:576
McCoy RE, Dimock AW (1972) Relationship of temperature and humidity to development of Mycosphaerella lesions on chrysanthemum. Phytopathol 62:1195–1196
McCoy RE, Dimock A (1973) Environmental factors regulating ascospore discharge by Mycosphaerella ligulicola. Phytopathol 63:586–589
McCoy RE, Horst R, Dimock A (1972) Environmental factors regulating sexual and asexual reproduction by Mycosphaerella ligulicola. Phytopathol 62:1188–95
McDonald M, Razavi M, Friesen T, Brunner P, McDonald B (2012) Phylogenetic and population genetic analyses of Phaeosphaeria nodorum and its close relatives indicate cryptic species and an origin in the Fertile Crescent. Fungal Genet Biol 49:882–95
Müller E, Von Arx JA (1962) Die Gattungen der didymosporen Pyrenomyceten. Beitr Kryptogamenflora Schweiz 11:1–922
Oxenham BL (1963) Report of the Plant Pathology Section. Report for the Department of Agriculture, Queensland, Australia
Peregrine W, Watson D (1964) Annual report of the plant pathology section. Department of Agriculture, Tanganyika
Pérez C, De Beer Z, Altier N, Wingfield M, Blanchette R (2008) Discovery of the eucalypt pathogen Quambalaria eucalypti infecting a non-Eucalyptus host in Uruguay. Australas Plant Pathol 37:600–4
Pethybridge SJ, Hay FS (2001) Influence of Phoma ligulicola on yield, and site factors on disease development, in Tasmanian pyrethrum crops. Australas Plant Pathol 30:17–20
Pethybridge SJ, Wilson C (1998) Confirmation of ray blight disease of pyrethrum in Australia. Australas Plant Pathol 27:45–8
Pethybridge SJ, Scott J, Hay F (2004) Genetic relationships among isolates of Phoma ligulicola from pyrethrum and chrysanthemum based on ITS sequences and its detection by PCR. Australas Plant Pathol 33:173–81
Pethybridge SJ, Esker P, Hay F, Wilson C, Nutter FW Jr (2005a) Spatiotemporal description of epidemics caused by Phoma ligulicola in Tasmanian pyrethrum fields. Phytopathol 95:648–58
Pethybridge SJ, Hay FS, Wilson CR, Groom T (2005b) Development of a fungicide-based management strategy for foliar disease caused by Phoma ligulicola in Tasmanian pyrethrum fields. Plant Dis 89:1114–20
Pethybridge SJ, Hay FS, Jones S, Wilson C, Groom T (2006) Seedborne infection of pyrethrum by Phoma ligulicola. Plant Dis 90:891–7
Pethybridge SJ, Esker P, Dixon P, Dixon P, Hay F, Groom T, Wilson C, Nutter FW Jr (2007a) Quantifying loss caused by ray blight disease in Tasmanian pyrethrum fields. Plant Dis 91:1116–21
Pethybridge SJ, Hay F, Esker P, Wilson C, Nutter FW Jr (2007b) Use of a multispectral radiometer for noninvasive assessments of foliar disease caused by ray blight in pyrethrum. Plant Dis 91:1397–406
Pethybridge SJ, Hay FS, Clarkson R, Groom T, Wilson C (2008a) Host range of Australian Phoma ligulicola var. inoxydablis isolates from pyrethrum. J Phytopathol 156:506–8
Pethybridge SJ, Hay FS, Esker PD, Gent DH, Wilson CR, Groom T, Nutter FW Jr (2008b) Diseases of pyrethrum in Tasmania: challenges and prospects for management. Plant Dis 92:1260–72
Pethybridge SJ, Hay FS, Esker PD, Groom T, Wilson C, Nutter FW (2008c) Visual and radiometric assessments for yield losses caused by ray blight in pyrethrum. Crop Sci 48:343–52
Pethybridge SJ, Hay FS, Groom T, Wilson CR (2008d) Improving fungicide–based management of ray blight disease in Tasmanian pyrethrum fields. Plant Dis 92:887–95
Pethybridge SJ, Gent DH, Esker PD, Turechek WW, Hay FS, Nutter FW Jr (2009) Site-specific risk factors for ray blight in Tasmanian pyrethrum fields. Plant Dis 93:229–7
Pethybridge SJ, Ngugi H, Hay F (2010) Use of survival analysis to assess management options for ray blight in Australian pyrethrum fields. Plant Pathol 59:480–91
Pethybridge SJ, Gent DH, Hay FS (2011) Epidemics of ray blight on pyrethrum are linked to seed contamination and overwintering inoculum of Phoma ligulicola var. inoxydabilis. Phytopathol 101:1112–21
Pethybridge SJ, Scott JB, Hay FS (2012) Lack of evidence for recombination or spatial structure in Phoma ligulicola var. inoxydabilis populations from Australian pyrethrum fields. Plant Dis 96:746–51
Pethybridge SJ, Gent DH, Groom T, Hay FS (2013) Minimizing crop damage through understanding relationships between pyrethrum phenology and ray blight disease severity. Plant Dis 97:1431–7
Punithalingam E (1980) Didymella chrysanthemi. C.M.I. Descriptions of Pathogenic Fungi and Bacteria no. 662:1–3
Richardson MJ (1979) An annotated list of seed-borne diseases. C.M.I. Phytopathol Pap 23:1–320
Robinson RA (1963) Diseases of pyrethrum in Kenya. East Afr Agric For J 28:164–167
Rossi V, Candresse T, Jeger MJ, Manceau C, Urek G, Stancanelli G (2014) Diagnosis of plant pathogens and implications for plant quarantine: a risk assessment perspective. In: Gullino ML, Bonants PJ (eds) Detection and diagnostics of plant pathogens. Springer, Netherlands, pp 167–193
Schadler DL, Bateman DF (1974) Ascochyta chrysanthemi toxin: production and properties. Phytopathol 64:779–84
Schadler DL, Bateman DF (1975) Ascochyta chrysanthemi toxin: purification and partial characterization. Phytopathol 65:912–917
Shaw DE (1984) Micro-organisms in Papua New Guinea. Department of Primary Industry, Res. Bull. No. 33, Port Moresby, Papua New Guinea
Simmonds JH (1996) Host index of plant disease in Queensland. Queensland Department of Primary Industries, Brisbane
Stevens FL (1907) The chrysanthemum ray blight. Bot Gaz 44:241–258
Tassi F (1900) Novae micromycetum species descriptae et iconibus iIIustratae. Bollettino del laboratorio ed orto botanico della r. Uniuersita di Siena 3:117–132
Tomilin BA (1979) Key to fungi of the genus Mycosphaerella Iohans., 320 pp. Leningrad: U.S.S.R. (original not seen, from Index of Fungi 4, 598, 1980)
Vaghefi N, Pethybridge SJ, Ford R, Nicolas ME, Crous PW, Taylor PWJ (2012) Stagonosporopsis spp. associated with ray blight disease of Asteraceae. Australas Plant Pathol 41:675–686
Vaghefi N, Ades PK, Hay FS, Pethybridge SJ, Ford R, Taylor PWJ (2015a) Identification of the MAT1 locus in Stagonosporopsis tanaceti, and exploring its potential for sexual reproduction in Australian pyrethrum fields. Fungal Biol 119:408–419
Vaghefi N, Hay FS, Ades PK, Pethybridge SJ, Ford R, Taylor PWJ (2015b) Rapid changes in the genetic composition of Stagonosporopsis tanaceti population in Australian pyrethrum fields. Phytopathol 105:358–369
Vaghefi N, Hay FS, Pethybridge SJ, Ford R, Taylor PWJ (2016) Development of a multiplex PCR diagnostic assay for the detection of Stagonosporopsis species associated with ray blight of Asteraceae. Eur J Plant Pathol. doi:10.1007/s10658-016-0944-4
Van der Aa H, Noordeloos M, de Gruyter J (1990) Species concepts in some larger genera of the Coelomycetes. Stud Mycol 32:3–19
Van Steekelenburg PN (1978) Chemical control of Didymella bryoniae in cucumbers. Netherlands J Plant Pathol 84:27–34
Vlasveld WPN (1977) The use of systemic fungicides in Dutch horticulture. Eur J Plant Pathol 83:297–303
Voglino P (1902) Sopra una malattia dei crisantemi coltivati. Malp ighia is, 329–341
Von Mueller F (1895) Select extra-tropical plants, readily eligible for industrial culture or naturalisation, 9th edn. Government Printer, Melbourne
Walker J, Baker KF (1983) The correct binomial for the chrysanthemum ray blight pathogen in relation to its geographical distribution. Trans Br Mycol Soc 80:31–38
Wingfield BD, Ades PK, Al-Naemi FA, Beirn LA, Bihon W, Crouch JA, de Beer ZW, De Vos L, Duong TA, Fields CJ, Fourie G et al (2015) IMA Genome-F 4: draft genome sequences of Chrysoporthe austroafricana, Diplodia scrobiculata, Fusarium nygamai, Leptographium lundbergii, Limonomyces culmigenus, Stagonosporopsis tanaceti, and Thielaviopsis punctulata. IMA Fungus 6:233–248
Woudenberg JH, De Gruyter J, Crous PW, Zwiers LH (2012) Analysis of the mating-type loci of co-occurring and phylogenetically related species of Ascochyta and Phoma. Mol Plant Pathol 13:350–62
Zaffarano PL, McDonald BA, Zala M, Linde C (2006) Global hierarchical gene diversity analysis suggests the fertile crescent is not the center of origin of the barley scald pathogen Rhynchosporium secalis. Phytopathol 96:941–50
Acknowledgments
We thank Mr Tim Groom, Botanical Resources Australia – Agricultural Services Pty. Ltd., for constructive discussions. This project was supported by Botanical Resources Australia – Agricultural Services Pty. Ltd. The first author gratefully acknowledges the financial support from the Melbourne International Research Scholarship (MIRS) and Melbourne International Fee Remission Scholarship (MIFRS) awarded by The University of Melbourne.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaghefi, N., Pethybridge, S.J., Hay, F.S. et al. Revisiting Stagonosporopsis species associated with chrysanthemum and pyrethrum ray blight. Australasian Plant Pathol. 45, 561–570 (2016). https://doi.org/10.1007/s13313-016-0446-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-016-0446-z