Abstract
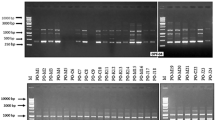
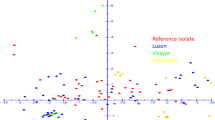
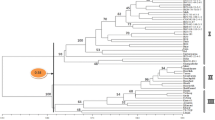
Rice blast, caused by Magnaporthe oryzae is one of the most destructive rice diseases worldwide. The genetic variability of 55 M. oryzae isolates from Iran and 32 isolates from Uruguay was analyzed using amplified fragment length polymorphism (AFLP). Cluster analysis using different grouping methods and similarity coefficients, based on the AFLP data from 679 monomorphic and polymorphic bands generated with eight primer combinations, was performed. The resulted grouping of the isolates revealed 4 separate AFLP lineages among a total of 87 isolate, designated as A1, A2, B, and C. Within each AFLP lineage, three or more clones were detected with a genetic similarity of 100 %. Overall genetic similarity was greater than 78 % between Iranian and Uruguayan populations. Evidence of gene flow between the Iranian and Uruguayan populations obtained from indica rice cultivars was observed as one Iranian isolate grouped lineage 1A together with Urugyayan isolates. Analysis of molecular variance (AMOVA) revealed that rice varietal type, sampling year and geographic region were the dominant factors determining genetic structure of M. oryzae populations; but rice cultivar had not significant effect.

Similar content being viewed by others
References
Borromeo ES, Nelson RJ, Bonman JM, Leung H (1993) Genetic differentiation among isolates of Pyricularia infecting rice and weed hosts. Phytopathology 83:393–399
Ceresini PC, Shew HD, Vilgalys RJ, Cubeta MA (2002) Genetic diversity of Rhizoctonia solani AG-3 from potato and tobacco in North Carolina. Mycologia 94:437–449
Chen DH, Zeigler RS, Leung H, Nelson RJ (1995) Population structure of Pyricularia grisea at two screening sites in the Philippines. Phytopathology 85:1011–1020
Consolo VF, Cordo CA, Salerno GL (2005) Mating-type distribution and fertility status in Magnaporthe grisea populations from Argentina. Mycopathologia 160:285–290
Consolo VF, Cordo CA, Salerno GL (2008) DNA fingerprinting and pathotype diversity of Magnaporthe oryzae populations from Argentina. Australas Plant Pathol 37:357–364
Correll JC, Harp TL, Guerber JC, Zeigler RS, Liu B, Cartwright RD, Lee FN (2000) Characterization of Pyricularia grisea in the United States using independent genetic and molecular markers. Phytopathology 90:1396–1404
Darrasse A, Darsonval A, Boureau T, Brisset MN, Durand K, Jacques MA (2010) Transmission of plant-pathogenic bacteria by nonhost seeds without induction of an associated defense reaction at emergence. Appl Environ Microbiol 76:6787–6796
Dayakar BV, Narayanan NN, Gnanamanickam SS (2000) Cross compatibility and distribution of mating type alleles of the rice blast fungus Magnaporthe grisea in India. Plant Dis 84:700–704
Devulapalle KS, Suryanarayanen S (1995) Cross-incompatibility among Indian isolates of Pyricularia grisea. Plant Dis 79:779–781
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26:297–302
Don LD, Kusaba M, Urashima AS, Tosa Y, Nakayashiki H, Mqayama S (1999) Population structure of the rice blast fungus in Japan examined by DNA fingerprinting. Ann Phytopathol Soc Jpn 65:15–24
Fahleson J, Lagercrantz U, Steventon LA, Dixelius C (2003) Evaluation of genetic variation among Verticillium isolates using AFLP analysis. Eur J Plant Pathol 109:361–371
George MLC, Nelson RJ, Zeigler RS, Leung H (1998) Rapid population analysis of Magnaporthe grisea by using rep-PCR and endogenous repetitive DNA sequence. Phytopathology 88:223–228
Han SS, Ra DS, Nelson RJ (1993) Comparisons of phylogenetic trees and pathotypes of Magnaporthe oryzae in Korea. J Agric Sci 35:315–323
Herrera TG, Duque DP, Almeida IP, Nunez GT, Pieters AJ, Martinez CP, Tohme JM (2008) Assessment of genetic diversity in Venezuelan rice cultivars using simple sequence repeats markers. Electron J Biotechnol 11:1–14
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bulletin de la Société vaudoise des sciences naturelles 44:223–270
Javan-Nikkhah M, McDonald BA, Banke S, Hedjaroude GA (2004) Genetic structure of Iranian Pyricularia grisea populations based on rep-PCR fingerprinting. Eur J Plant Pathol 110:909–919
Jeung JU, Hwang HG, Moon HP, Jena KK (2005) Fingerprinting temperate japonica and tropical indica rice genotypes by comparative analysis of DNA markers. Euphytica 146:239–251
Jing Z, ShuJie F, JiangQiao C, Ling W, Fei L, Qiang HP (2009) Distribution of mating type and sexual status in Chinese rice blast populations. Plant Dis 93:238–242
Jones RK, Belmar SB (1989) Characterization and pathogenicity of Rhizoctonia spp. isolates from rice, soybean, and other crops grown in rotation with rice in Texas. Plant Dis 73:1004–1010
Kumar J, Nelson RJ, Zeigler RS (1999) Population structure and dynamics of Magnaporthe grisea in the Indian Himalayas. Genetics 152:971–984
Leung H, Taga M (1988) Magnaporthe grisea (Pyricularia grisea), the blast fungus. Adv Plant Pathol 6:175–188
Levy M, Romao J, Marchetti MA, Harmer JE (1991) DNA fingerprinting with a dispersed repeated sequence resolves pathotypic diversity in the rice blast fungus. Plant Cell 3:95–102
Levy M, Correa-Victoria FJ, Zeigler RS, Xu S, Hamer JE (1993) Genetic diversity of the rice blast fungus in a disease nursery in Colombia. Phytopathology 83:1427–1433
Linde CC, Zala M, Paulraj RSD, McDonald BA, Gnanamanickam S (2005) Population structure of the rice sheath blight pathogen Rhizoctonia solani AG-1IA from India. Eur J Plant Pathol 112:113–121
Lynch M, Milligan B (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
McDonald BA, Linde CC (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Ann Rev Phytopathol 40:349–379
Motallebi P, Javan-Nikkhah M, Okhovvat SM, Fotouhifar KB, Bargnil M (2009) Vegetative compatibility groups within Iranian populations of Magnaporthe grisea species complex from rice and some grasses. J Plant Pathol 91:469–473
Moumeni A, Leung H (2003) Genetic and molecular dissection of blast resistance in rice using RFLP, simple sequence repeats and defense-related candidate gene markers. Iran J Biotechnol 1:47–58
Muller UG, Wolfenbarger LL (1999) AFLP genotyping and fingerprinting. Trends Ecol Evol 14:389–394
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A 76:5267–5273
Ninh Thuan N, Bigirimana J, Roumen E, Van Der Straeten D, Höfte M (2006) Molecular and pathotypes analysis of rice blast fungus in North Vietnam. Eur J Plant Pathol 114:381–396
Noguchi MT, Yasuda N, Fujita Y (2007) Fitness Characters in parasexual recombinants of the rice blast fungus, Magnaporthe oryzae. Jpn Agric Res Quart 41:123–131
Oka HI (1988) Origin of cultivated rice. Elsevier/Jpn. Sci. Soc. Press, Amsterdam/Tokyo, 254 pp
Park SU, Milgroom MG, Han SS, Kang S, Lee YH (2003) Diversity of pathotypes and DNA fingerprint haplotypes in populations of Magnaporthe grisea in Korea over two decades. Phytopathology 93:1378–1385
Piotti E, Rigano MM, Rodino D, Rodolfi M, Castiglione A, Picco M, Sala F (2005) Genetic structure of Pyricularia grisea (Cooke) Sacc. isolates from Italian paddy fields. J Phytopathol 153:80–86
Prabhu AS, Araujo LG, Silva GB, Trindade MG (2007) Virulence and rep-PCR analysis of Pyricularia grisea isolates from two Brazilian upland rice cultivars. Fitopatol Bras 32:13–20
Rathour R, Singh BM, Plaha P (2006) Virulence structure of the Magnaporthe grisea rice population from the Northwestern Himalayas. Phytoparasitica 34:281–291
Rogers SO, Rogers MAM (1999) Gene flow in fungi. In: Worrall JJ (ed) Structure and dynamics of fungal populations. J Kluwer Academic, Dordrecht, pp 97–121
Rohlf FJ (2000) NTSYS-pc. Numerical taxonomy and multivariate analysis system. Version 2.1. Exeter Software, Setauket
Roumen E, Levy M, Notteghem JL (1997) Characterization of the European pathogen population of Magnaporthe grisea by DNA fingerprinting and pathotype analysis. Eur J Plant Pathol 103:363–371
Schneider S, Roessli D, Excoffier L (2000) Arlequin: A software for population genetics data analysis, version 2.0. University of Geneva, Switzerland
Simmons HE, Holmes EC, Gildow FE, Bothe-Goralczyk MA, Stephenson AG (2011) Experimental verification of seed transmission of Zucchini yellow mosaic virus. Plant Dis 95:751–754
Sneath PH, Sokal RR (1973) Numerical taxonomy. W. H. Freeman and Co., San Francisco
Sokal RR, Michener CD (1958) A statistical method for evaluating systematic relationships. Univ Kansas Sci Bull 38:1409–1438
Suzuki F, Arai M, Yamaguchi J (2006) DNA fingerprinting of Pyricularia grisea by rep-PCR using single primers designed from the terminal inverted repeat of each of the transposable elements Pot2 and MGR586. J Gen Plant Pathol 72:314–317
Suzuki F, Arai M, Yamaguchi J (2007) Genetic analysis of Pyricularia grisea population by rep-PCR during development of resistance to scytalone dehydratase inhibitors of melanin biosynthesis. Plant Dis 91:176–184
Taheri P, Tarighi S (2012) Genetic and virulence analysis of Rhizoctonia spp. associated with sugar beet root and crown rot in the northeast region of Iran. Plant Dis 96:398–408
Taheri P, Bonecarrere V, Höfte M (2004) Characterization of a Pyricularia grisea population from Uruguay by molecular analysis. Commun Agric Appl Biol Sci 69:207–210
Taheri P, Gnanamanickam S, Höfte M (2007) Characterization, genetic structure, and pathogenicity of Rhizoctonia spp. associated with rice sheath diseases. Phytopathology 97:373–383
Takan JP, Chipili J, Muthumeenakshi S, Talbot NJ, Manyasa EO, Bandyopadhyay R, Sere Y, Nutsugah SK, Talhinhas P, Hossain M, Brown AE, Sreenivasaprasad S (2011) Magnaporthe oryzae populations adapted to finger millet and rice exhibit distinctive patterns of genetic diversity, sexuality and host interaction. Mol Biotechnol 50:145–158
Talbot NJ, Ebbole DJ, Hamer JE (1993) Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. The Plant Cell 5:1575–1590
Tosa Y, Uddin W, Viji G, Kang S, Mayama S (2007) Comparative genetic analysis of Magnaporthe oryzae isolates causing gray leaf spot of perennial ryegrass turf in the United States and Japan. Plant Dis 91:517–524
Tredway LP, Stevenson KL, Burpee LL (2005) Genetic structure of Magnaporthe grisea populations associated with St. augustinegrass and tall fescue in Georgia. Phytopathology 95:463–471
Turgay EB, Bakir M, Ozeren P, Katircioglu YZ, Maden S (2010) Detection of pathotypes and genetic diversity of Cercospora beticola. Plant Pathol J 26:306–312
Urashima AS, Silva CP (2011) Characterization of Magnaporthe grisea (Pyricularia grisea) from black oat in Brazil. J Phytopathol 159:789–795
Van de Peer Y, De Wachter R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci 10:569–570
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zebeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Zeigler RS, Thome J, Nelson R, Levy M, Correa-Victoria FJ (1994) Lineage exclusion: A proposal for linking blast population analysis to resistance breeding. In: Zeigler RS, Leong RS, Teng PS (eds) Rice blast disease. CABI/IRRI, Wallingford, pp 267–292
Zeigler RS, Cuoc LX, Scout RP, Bernardo MA, Chen DH, Valent B, Nelson RJ (1995) The relationship between lineage and virulence in Pyricularia grisea in the Philippines. Phytopathology 85:443–451
Zhao DL, Atlin GN, Bastiaans L, Spiertz JHJ (2006) Comparing rice germplasm groups for growth, grain yield and weed-suppressive ability under aerobic soil conditions. Weed Res 46:444–452
Zolan M, Pukkila P (1986) Inheritance of DNA methylation in Coprinus cinereus. Mol Cell Biol 6:195–200
Acknowledgments
This project was supported by funding from Ferdowsi University of Mashhad with project number 3/24096. Also, thanks to molecular biology group of Shahid Beheshti University (Iran) for assistance during this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taheri, P., Irannejad, A. Genetic structure of various Magnaporthe oryzae populations in Iran and Uruguay. Australasian Plant Pathol. 43, 287–297 (2014). https://doi.org/10.1007/s13313-013-0269-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-013-0269-0