Abstract
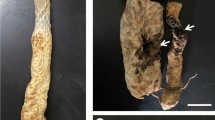
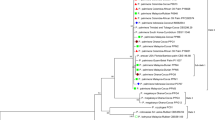
Lasiodiplodia theobromae is a plurivorous pathogen of tropical and subtropical woody and fruit trees. In 2010, an investigation of mango plantations in Egypt resulted in the isolation of 26 Lasiodiplodia isolates that, based on previous reports from literature, were tentatively identified as L. theobromae. The aim of this study was to clarify the taxonomy of these isolates based on morphology and DNA sequence data (ITS and TEF1-α). In addition to L. theobromae, a new species, namely L. egyptiacae, was identified. Furthermore, L. pseudotheobromae is also newly recorded on mango in Egypt. Pathogenicity tests with all recognised species showed that they are able to cause dieback disease symptoms on mango seedlings.




Similar content being viewed by others
References
Abdalla MA, Safie MH, El-Boghdady MM, Soltan HHM (2003) Fruit coating with certain plant oils for controlling post-harvest diseases of mangoes with special reference to stem end rot. Egypt J Appl Sci 18:116–136
Abdalla AEM, Darwish SM, Ayad EHE, El-Hamahmy RM (2007) Egyptian mango by-product 1. Compositional quality of mango seed kernel. Food Chem 103:1134–1140
Abd-El Ghani HS, Fatouh HM (2005) First record of sugar beet root rot disease caused by Botryodiplodia theobromae in Egypt. Egypt J Phytopathol 33(1):107–108
Abdollahzadeh J, Javadi A, Mohammadi Goltapeh E, Zare R, Phillips AJL (2010) Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 25:1–10
Abo-El-Dahab MK, El-Kazazz SA, Shoeib AA, El-Sheikh MA (1992) Biochemical changes in citrus fruits infected with Botryodiplodia theobromae. J Agric Sci Mansoura Univ 17:3525–3532
Al Adawi AO, Deadman ML, Al Rawahi AK, Khan AJ, Al Maqbali YM (2003) Diplodia theobromae associated with sudden decline of mango in the Sultanate of Oman. Plant Pathol 52:419
Alves A, Crous PW, Correia A, Phillips AJL (2008) Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers 28:1–13
Begoude BAD, Slippers B, Wingfield MJ, Roux J (2009) Botryosphaeriaceae associated with Terminalia catappa in Cameron, South Africa and Madagascar. Mycol Prog 9:101–123
Burgess TI, Barber A, Mohali S, Pegg G, De Beer W, Wingfield MJ (2006) Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 98(2):423–435
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91(3):553–556
Charles ML (1970) Effects of temperatures on conidium characteristics of Ulocladium chartum and Stemphylium floridanum. Mycologia 62(5):1071–1076
Chen SF, Pavlic D, Roux J, Slippers B, Xie YJ, Wingfield MJ, Zhou XD (2011) Characterization of Botryosphaeriaceae from plantation-grown Eucalyptus species in South China. Plant Pathol 60:739–751
Clendinin L (1896) Lasiodiplodia Ellis. & Everh. n. gen. Bot Gaz 21:92–93
Crous PW, Groenewald JZ (2005) Hosts, species and genotypes: opinions versus data. Australas Plant Pathol 34:463–470
Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G (2004) MycoBank: an online initiative to launch mycology into the 21st century. Stud Mycol 50:19–22
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas FO, Phillips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
Damm U, Crous PW, Fourie PH (2007) Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora sp. nov. Mycologia 99(5):664–680
de Hoog GS, Gerrits van den Ende AHG (1998) Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41:183–189
de Oliveira Costa VS, Michereff SJ, Martins RB, Gava CAT, Mizubuti ESG, Câmara MPS (2010) Species of Botryosphaeriaceae associated on mango in Brazil. Eur J Plant Pathol 127:509–519
de Wet J, Burgess T, Slippers B, Preisig O, Wingfield BD, Wingfield MJ (2003) Multiple gene genealogies and microsatellite markers reflect relationships between morphotypes of Sphaeropsis sapinea and distinguish a new species of Diplodia. Mycol Res 107:557–566
Denman S, Crous PW, Taylor JE, Ka JC, Pacose I, Wingfield MJ (2000) An overview of the taxonomic history of Botryosphaeria and re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Stud Mycol 45:129–140
Denman S, Crous PW, Groenewald JZ, Slippers B, Wingfield BD, Wingfield MJ (2003) Circumscription of Botryosphaeria species associated with Proteaceae based on morphology and DNA sequence data. Mycologia 95:294–307
Diab MM, Kahlil I, Dawood NA, El-Assiuty EM (1984) Ear and grain rot of maize caused by Botryodiplodia theobromae pathogens in Egypt. Minufiya J Agric Res 9:129–138
El Tomi AL (1953) Subtropical fruit industry in Egypt. Proc Fla State Hortic Soc 66:195–198
El-Goorani MA, El Meleigi MA (1972) Dieback of grapevine by Botryodiplodia theobromae Pat. in Egypt. Phytopathol Mediterr 11:210–211
El-Soukkary FAH, El-Sahn MA, Mohamed HMA (2000) Physico-chemical and nutritional evaluation of mango seed kernel and its utilization for pan bread supplementation. Zagazig J Agric Res 27:1319–1342
Glienke C, Pereira OL, Stringari D, Fabris J, Kava-Cordeiro V, Galli-Terasawa L, Cunnington J, Shivas RG, Groenewald JZ, Crous PW (2011) Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot. Persoonia 26:47–56
Gueidan C, Roux C, Lutzoni F (2007) Using multigene phylogeny analysis to assess generic delineation and character evolution in Verrucariaceae (Verrucariales, Ascomycota). Mycol Res 111:1145–1168
Haggag WM, Nofal MA (2006) Improving the biological control of Botryodiplodia disease on some Annona cultivars using single or multi-bioagents in Egypt. Biol Control 38:341–349
Haggag WM, AbouRayya MSM, Kasim NE (2007) First report of a canker disease of walnut caused by Botryodiplodia theobromae in Egypt. Plant Dis 91:226
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192
Jacobs R (2002) Characterization of Botryosphaeria species from mango in South Africa. Dissertation, University of Pretoria
Johnson GI (1992) Biology and control of stem end rot pathogens of mango. Dissertation, University of Queensland
Johnson GI, Cooke AW, Mead AJ, Wells IA (1991) Stem end-rot of mango in Australia: causes and control. Acta Hortic 219:288–295
Johnson GI, Cooke T, Mead A (1993) Infection and quiescent of mango stem-end rot pathogens. Acta Hortic 341:329–336
Katoh K, Toh H (2010) Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26:1899–1900
Khanzada MA, Lodhi A, Shahzad S (2004a) Mango dieback and gummosis in Sindh, Pakistan caused by Lasiodiplodia theobromae. Plant Health Prog. doi:10.1094/PHP-2004-0302-01-DG
Khanzada MA, Lodhi AM, Shahzad S (2004b) Pathogenicity of Lasiodiplodia theobromae and Fusarium solani on mango. Pak J Bot 36(1):181–189
Kim YK, Xiao CL, Rogers JD (2005) Influence of culture media and environmental factors on mycelial growth and pycnidial production of Sphaeropsis pyriputrescens. Mycologia 97(1):25–32
Knight RJ (1993) Evaluating important fruit characters in mango germplasm. Fruit Var J 47(1):25–30
Larget B, Simon D (1999) Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Mol Biol Evol 16:750–759
Marincowitz S, Groenewald JZ, Wingfield MJ, Crous PW (2008) Species of Botryosphaeriaceae occurring on Proteaceae. Persoonia 21:111–118
Mason-Gamer R, Kellogg E (1996) Testing for phylogenetic conflict among molecular datasets in the tribe Tiriceae (Graminae). Syst Biol 45:524–545
Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci U S A 95:2044–2049
Patouillard N, De Lagerheim G (1892) Champignons de l’equateur (Pugillus II). Bull Soc Mycol Fr 8:113–140
Pavlic D, Slippers B, Coutinho TA, Gryzenhout M, Wingfield MJ (2004) Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Stud Mycol 50:313–322
Pavlic D, Wingfield MJ, Barber P, Slippers B, Hardy GESJ, Burgess TI (2008) Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 100(6):851–866
Pennycook SR, Samuels GJ (1985) Botryosphaeria and Fusicoccum species associated with ripe fruit rot of Actinidia deliciosa (kiwifruit) in New Zealand. Mycotaxon 24:445–458
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21:29–55
Ploetz RC (2004) The major diseases of mango: strategies and potential for sustainable management. Proc Mango Acta Hort 645:137–150
Ploetz RC, Benscher D, Vázquez A, Colls A, Nagel J, Schaffer B (1996a) Mango decline: research in Florida on an apparently widespread disease complex. Proceeding of International Mango Symposium. Acta Hortic 455:547–553
Ploetz RC, Benscher D, Vázquez A, Colls A, Nagel J, Schaffer B (1996b) A re-examination of mango decline in Florida. Plant Dis 80:664–668
Punithalingam E (1976) Botryodiplodia theobromae. CMI descriptions of pathogenic fungi and bacteria, No.519. Commonwealth Mycological Institute, Key, Surrey, England
Punithalingam E (1980) Plant diseases attributed to Botryodiplodia theobromae Pat. J. Carmer. Vaduz
Ragab MM, Sabet KA, Dawood NA (1971) Botryodiplodia theobromae Pat. The cause of fruit rot and die back of mango in A.R.E. Agric Res Rev 49:81–97
Ramos LJ, Lara SP, McMillan RT, Narayanan KR (1991) Tip die back of mango (Mangifera indica) caused by Botryosphaeria ribis. Plant Dis 75:315–318
Rayner RW (1970) A mycological colour chart. CMI and British Mycological Society, Kew, Surrey, UK
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Sakalidis ML, Ray JD, Lanoiselet V, Hardy GES, Burgess TI (2011) Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley region of Western Australia. Eur J Plant Pathol 130:379–391
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and their impact. Fungal Biol Rev 21:90–106
Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ (2004a) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96(1):83–101
Slippers B, Fourie G, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ (2004b) Multiple gene sequences delimit Botryosphaeria australis sp. nov. from B. lutea. Mycologia 96(5):1030–1041
Slippers B, Johnson GI, Crous PW, Coutinho TA, Wingfield B, Wingfield MJ (2005) Phylogenetic and morphological re-evolution of the Botryosphaeria species causing diseases of Mangifera indica. Mycologia 97(1):99–110
Smith H, Crous PW, Wingfield MJ, Coutinho TA, Wingfield BD (2001) Botryosphaeria eucalyptorum sp. nov., a new species in the B. dothidea-complex on Eucalyptus in South Africa. Mycologia 93(2):277–285
Summerbell RC, Krajden S, Levine R, Fuksa M (2004) Subcutaneous phaeohyphomycosis caused by Lasiodiplodia theobromae and successfully treated surgically. Med Mycol 42:543–547
Swofford DL (2001) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinaur Associates, Sunderland
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. San Diego, California, pp 315–322
Zhao JP, Lu Q, Liang J, Decock C, Zhang XY (2010) Lasiodiplodia pseudotheobromae, a new record of pathogenic fungus from some subtropical and tropical trees in southern China. Cryptogam Mycol 31:431–439
Acknowledgments
This work was partially funded by the CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, the Netherlands, University of Catania, Italy, and the Plant Pathology Research Institute, Giza, Egypt. The first author would like to thank all the staff at the CBS for their guidance and technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ismail, A.M., Cirvilleri, G., Polizzi, G. et al. Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australasian Plant Pathol. 41, 649–660 (2012). https://doi.org/10.1007/s13313-012-0163-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-012-0163-1