Abstract
A major goal of contemporary epilepsy research is the identification of therapies to prevent the development of recurrent seizures in individuals at risk, including those with brain injuries, infections, or neoplasms; status epilepticus; cortical dysplasias; or genetic epilepsy susceptibility. In this review we consider the evidence largely from preclinical models for the antiepileptogenic activity of a diverse range of potential therapies, including some marketed antiseizure drugs, as well as agents that act by immune and inflammatory mechanisms; reduction of oxidative stress; activation of the mammalian target of rapamycin or peroxisome proliferator-activated receptors γ pathways; effects on factors related to thrombolysis, hematopoesis, and angiogenesis; inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reducatase; brain-derived neurotrophic factor signaling; and blockade of α2 adrenergic and cannabinoid receptors. Antiepileptogenesis refers to a therapy of which the beneficial action is to reduce seizure frequency or severity outlasting the treatment period. To date, clinical trials have failed to demonstrate that antiseizure drugs have such disease-modifying activity. However, studies in animal models with levetiracetam and ethosuximide are encouraging, and clinical trials with these agents are warranted. Other promising strategies are inhibition of interleukin 1β signaling by drugs such as VX-765; modulation of sphingosine 1-phosphate signaling by drugs such as fingolimod; activation of the mammalian target of rapamycin by drugs such as rapamycin; the hormone erythropoietin; and, paradoxically, drugs such as the α2 adrenergic receptor antagonist atipamezole and the CB1 cannabinoid antagonist SR141716A (rimonabant) with proexcitatory activity. These approaches could lead to a new paradigm in epilepsy drug therapy where treatment for a limited period prevents the occurrence of spontaneous seizures, thus avoiding lifelong commitment to symptomatic treatment.
Similar content being viewed by others
References
Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 2011;52:657–678.
Wilcox KS, Dixon-Salazar T, Sills GJ, et al. Issues related to development of new antiseizure treatments. Epilepsia 2013;54(Suppl. 4):24–34.
Perucca E, French J, Bialer M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol 2007;6:793–804.
Löscher W, Klitgaard H, Twyman RE, et al. New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov 2013;12:757–776.
Schmidt D, Friedman D, Dichter MA. Anti-epileptogenic clinical trial designs in epilepsy: Issues and options. Neurotherapeutics 2014. doi: 10.1007/s13311-013-0252-z.
Pitkänen A. Therapeutic approaches to epileptogenesis-hope on the horizon. Epilepsia 2010;51(Suppl. 3):2–17.
Nadler JV. Plasticity of glutamate synaptic mechanisms. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper's basic mechanisms of the epilepsies. 4th ed. National Center for Biotechnology Information, Bethesda, MD, 2012, available at: http://www.ncbi.nlm.nih.gov/books/NBK98204. Accessed March 16, 2014.
Porter RJ, Dhir A, Macdonald RL, et al. Mechanisms of action of antiseizure drugs. Handb Clin Neurol 2012;108:663–681.
Margineanu DG, Klitgaard H. Mechanisms of drug resistance in epilepsy: relevance for antiepileptic drug discovery. Expert Opin Drug Discov 2009;4:23–32.
Rogawski MA. Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics 2009;6:344–351.
Klitgaard H, Matagne A, Schachter S, et al. Animal and translational models of the epilepsies. In: Animal and translational models of behavioral disorders. McArthur RA, Borsini F (eds) XXX. Elsevier, New York, 2008, pp. 311–335.
White HS, Löscher W. Searching for the ideal antiepileptogenic agent in experimental models: single treatment versus combinatorial treatment strategies. Neurotherapeutics 2014. doi: 10.1007/s13311-013-0250-1
Silver JM, Shin C, McNamara JO. Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilepsy. Ann Neurol 1991;29:356–363.
Löscher W, Hönack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther 1998;284:474–479.
Stratton SC, Large CH, Cox B, et al. Effects of lamotrigine and levetiracetam on seizure development in a rat amygdala kindling model. Epilepsy Res 2003;53:95–106.
Blumenfeld H, Klein JP, Schridde U, et al. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia 2008;49:400–409.
Russo E, Citraro R, Scicchitano F, et al. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia 2010;51:1560–1569.
Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia 2009;50(Suppl. 2):10–13.
Löscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: Experimental approaches and translational research. Pharmacol Rev 2010;62:668–700.
Milligan TA, Hurwitz S, Bromfield EB. Efficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgery. Neurology 2008;71:665–669.
Klein P, Herr D, Pearl PL, et al. Results of phase 2 safety and feasibility study of treatment with levetiracetam forprevention of posttraumatic epilepsy. Arch Neurol 2012;69:1290–1295.
Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 2001;276:36734–36741.
Jessberger S, Nakashima K, Clemenson GD Jr, et al. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci 2007;27:5967–5975.
Holmes KH, Bilkey DK, Laverty R, et al. The N-methyl-D-aspartate antagonists aminophosphonovalerate and carboxypiperazinephosphonate retard the development and expression of kindled seizures. Brain Res 1990;506:227–235.
Dürmüller N, Craggs M, Meldrum BS. The effect of the non-NMDA receptor antagonist GYKI 52466 and NBQX and the competitive NMDA receptor antagonist D-CPPene on the development of amygdala kindling and on amygdala-kindled seizures. Epilepsy Res 1994;17:167–174.
Bolanos AR, Sarkisian M, Yang Y, et al. Comparison of valproate and phenobarbital treatment after status epilepticus in rats. Neurology 1998;51:41–48.
Brandt C, Gastens AM, Sun M, et al. Treatment with valproate after status epilepticus: effect on neuronal damage, epileptogenesis, and behavioral alterations in rats. Neuropharmacology 2006;51:789–804.
Klitgaard HV, Matagne AC, Vanneste-Goemaere J, et al. Effects of prolonged administration of levetiracetam on pilocarpine-induced epileptogenesis in rat. Epilepsia 2001;42(Suppl. 7):114.
Schmidt D. Is antiepileptogenesis a realistic goal in clinical trials? Concerns and new horizons. Epileptic Disord 2012;14:105–113.
Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nature Med 2004;10, 685–692.
van Luijtelaar G, Mishra AM, Edelbroek P, et al. Anti-epileptogenesis: Electrophysiology, diffusion tensor imaging and behavior in a genetic absence model. Neurobiol Dis 2013;60:126–138.
Dezsi G, Ozturk E, Stanic D, et al. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia 2013;54:635–643.
Russo E, Citraro R, Scicchitano F, et al. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia 2011;52:1341–1350.
Becker AJ, Pitsch J, Sochivko D, et al. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neuroscience 2008;28:13341–13353.
Amano K, Hamada K, Yagi K, et al. Antiepileptic effects of topiramate on amygdaloid kindling in rats. Epilepsy Res 1998;31:123–128.
Mazarati A, Shin D, Auvin S, et al. Age-dependent effects of topiramate on the acquisition and the retention of rapid kindling. Epilepsia 2007;48:765–773.
Sankar R, Auvin S, Kwon YS, et al. Evaluation of development-specific targets for antiepileptogenic therapy using rapid kindling. Epilepsia 2010;51(Suppl. 3):39–42.
DeLorenzo RJ, Morris A, Blair RE, et al. Topiramate is both neuroprotective and antiepileptogenic in the pilocarpine model of status epilepticus. Epilepsia 2002;43(Suppl. 7):15.
Kudin AP, Debska-Vielhaber G, Vielhaber S, et al. The mechanism of neuroprotection by topiramate in an animal model of epilepsy. Epilepsia 2004;45:1478–1487.
Suchomelova L, Baldwin RA, Kubova H, et al. Treatment of experimental status epilepticus in immature rats: dissociation between anticonvulsant and antiepileptogenic effects. Pediatr Res 2006;59:237–243.
Rigoulot MA, Koning E, Ferrandon A, et al. Neuroprotective properties of topiramate in the lithium-pilocarpine model of epilepsy. J Pharmacol Exp Ther 2004;308:787–795.
François J, Koning E, Ferrandon A, et al. The combination of topiramate and diazepam is partially neuroprotective in the hippocampus but not antiepileptogenic in the lithium-pilocarpine model of temporal lobe epilepsy. Epilepsy Res 2006;72:147–163.
Shatskikh T, Zhao Q, Zhou JL, et al. Effect of topiramate on cognitive function and single units from hippocampal place cells following status epilepticus. Epilepsy Behav 2009;14:40–47.
Cha BH, Silveira DC, Liu X, et al. Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Res 2002;51:217–232.
Frisch C, Kudin AP, Elger CE, et al. Amelioration of water maze performance deficits by topiramate applied during pilocarpine-induced status epilepticus is negatively dose-dependent. Epilepsy Res 2007;73:173–180.
Niebauer M, Gruenthal M. Topiramate reduces neuronal injury after experimental status epilepticus. Brain Res 1999;837:263–269.
Hoover RC, Motta M, Davis J, et al. Differential effects of the anticonvulsant topiramate on neurobehavioral and histological outcomes following traumatic brain injury in rats. J Neurotrauma 2004;21:501–512.
Kouzounias K, Kimiskidis VK, Siozos T, et al. Topiramate promotes neurological recovery in a new model of traumatic brain injury in rats. Neuroscience 2011;183:171–177.
Alves OL, Doyle AJ, Clausen T, et al. Evaluation of topiramate neuroprotective effect in severe TBI using microdialysis. Ann NY Acad Sci 2003;993:25–34.
Dichter MA. Posttraumatic epilepsy: the challenge of translating discoveries in the laboratory to pathways to a cure. Epilepsia 2009;50(Suppl. 2):41–45.
Klitgaard H, Verdru P. Levetiracetam – the first SV2A ligand for the treatment of epilepsy. Expert Opin Drug Discov 2008;2:1537–1545.
Kaminski RM, Gillard M, Klitgaard H. Targeting SV2A for discovery of antiepileptic drugs. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies. 4th ed. National Center for Biotechnology Information, Bethesda, MD: National Center, 2012, available at http://www.ncbi.nlm.nih.gov/books/NBK98183. Accessed March 16, 2014.
Kaminski RM, Gillard M, Leclercq K, et al. Proepileptic phenotype of SV2A-deficient mice is associated with reduced anticonvulsant efficacy of levetiracetam. Epilepsia 2009;50:1729–1740.
Gu J, Lynch BA, Anderson D, et al. The antiepileptic drug levetiracetam selectively modifies kindling-induced alterations in gene expression in the temporal lobe of rats. Eur J Neurosci 2004;19:334–345.
Husum H, Bolwig TG, Sánchez C, et al. Levetiracetam prevents changes in levels of brain-derived neurotrophic factor and neuropeptide Y mRNA and of Y1- and Y5-like receptors in the hippocampus of rats undergoing amygdala kindling: implications for antiepileptogenic and mood-stabilizing properties. Epilepsy Behav 2004;5:204–215.
Vinogradova LV, van Rijn CM. Anticonvulsive and antiepileptogenic effects of levetiracetam in the audiogenic kindling model. Epilepsia 2008;49:1160–1168.
Matagne A, Margineanu DG, Kenda B, et al. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol 2008;154:1662–1671.
Yan HD, Ji-qun C, Ishihara K, et al. Separation of antiepileptogenic and antiseizure effects of levetiracetam in the spontaneously epileptic rat (SER). Epilepsia 2005;46:1170–1177.
Brandt C, Glien M, Gastens AM, et al. Prophylactic treatment with levetiracetam after status epilepticus: lack of effect on epileptogenesis, neuronal damage, and behavioral alterations in rats. Neuropharmacology 2007;53:207–221.
Mazarati AM, Baldwin RA, Klitgaard H, et al. Treatment with levetiracetam during the latent period after experimental status epilepticus reduces chronic spontaneous recurrent seizures. Epilepsia 2003;44(Suppl. 9):223.
Sugaya Y, Maru E, Kudo K, et al. Levetiracetam suppresses development of spontaneous EEG seizures and aberrant neurogenesis following kainate-induced status epilepticus. Brain Res 2010;1352:187–199.
Margineanu DG, Matagne A, Kaminski RM, et al. Effects of chronic treatment with levetiracetam on hippocampal field responses after pilocarpine-induced status epilepticus in rats. Brain Res Bull 2008;77:282–285.
Zhou JL, Zhao Q, Holmes GL. Effect of levetiracetam on visual-spatial memory following status epilepticus. Epilepsy Res 2007;73:65–74.
Pearl PL, McCarter R, McGavin CL, et al. Results of phase II levetiracetam trial following acute head injury in children at risk for posttraumatic epilepsy. Epilepsia 2013;54:135–137.
Szaflarski JP, Snagha KS, Lindsell CJ, et al. Prospective, randomized, single-binded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care 2010;12: 165–172.
Jehi LE, Irwin Al, Kayyali H, et al. Levetiracetam may favorably affect seizure outcome after temporal lobectomy. Epilepsia 2012;53:979–986.
Kobow K, Auvin S, Jensen F, et al. Finding a better drug for epilepsy: antiepileptogenesis targets. Epilepsia 2012;53:1868–1876.
Vezzani A, Balosso S, Ravizza T. Inflammation and epilepsy. Handb Clin Neurol 2012;107:163–175.
Devinsky O, Vezzani A, Najjar S, et al. Glia and epilepsy: excitability and inflammation. Trends Neurosci 2013;36:174–184.
Stack JH, Beaumont K, Larsen PD, et al. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J Immunol 2005;175:2630–2634.
Ravizza T, Lucas SM, Balosso S, et al. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia 2006;47:1160–1168.
Maroso M, Balosso S, Ravizza T, et al. Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 2011;8:304–315.
Noe FM, Polascheck N, Frigerio F, et al. Pharmacological blockade of IL-1β/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis 2013;59:183–193.
Ravizza T, Noé F, Zardoni D, et al. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol Dis 2008;31:327–333.
Viviani B, Bartesaghi S, Gardoni F, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 2003;23:8692–8700.
Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res 2013;103:2–30.
Fabene PF, Laudanna C, Constantin G. Leukocyte trafficking mechanisms in epilepsy. Mol Immunol 2013;55:100–104.
Fabene PF, Navarro Mora G, Martinello M, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med 2008;14:1377–1383.
Zattoni M, Mura ML, Deprez F, et al. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J Neurosci 2011;31:4037–4050.
Ravizza T, Gagliardi B, Noé F, et al. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis 2008;29:142–160.
Iyer A, Zurolo E, Spliet WG, et al. Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia 2010;51:1763–1773.
Kappos L, Bates D, Edan G, et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol 2011;10:745–758.
Ramirez P, Rettig MP, Uy GL, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood 2009;114:1340–1343.
Rojas A, Jiang J, Ganesh T, et al. Cyclooxygenase-2 in epilepsy. Epilepsia 2014;55:17–25.
Jung KH, Chu K, Lee ST, et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis 2006;23:237–246.
Holtman L, van Vliet EA, van Schaik R, et al. Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res 2009;84:56–66.
Holtman L, van Vliet EA, Edelbroek PM, et al. Cox-2 inhibition can lead to adverse effects in a rat model for temporal lobe epilepsy. Epilepsy Res 2010;91:49–56.
Polascheck N, Bankstahl M, Löscher W. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp Neurol 2010;224:219–233.
Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006;332:1302–1308.
Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation 2007;115:1634–1642.
Jiang J, Ganesh T, Du Y, et al. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci USA 2012;109:3149–3154.
Jiang J, Quan Y, Ganesh T, et al. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci USA 2013;110:3591–3596.
Kwon YS, Pineda E, Auvin S, et al. Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J Neuroinflammation 2013;10:30.
Soliven B, Miron V, Chun J. The neurobiology of sphingosine 1-phosphate signaling and sphingosine 1-phosphate receptor modulators. Neurology 2011;76(8 Suppl. 3):S9-14.
Gao F, Liu Y, Li X, et al. Fingolimod (FTY720) inhibits neuroinflammation and attenuates spontaneous convulsions in lithium-pilocarpine induced status epilepticus in rat model. Pharmacol Biochem Behav 2012;103:187–196.
Cardenas-Rodriguez N, Huerta-Gertrudis B, Rivera-Espinosa L, et al. Role of oxidative stress in refractory epilepsy: evidence in patients and experimental models. Int J Mol Sci 2013;14:1455–1476.
Rowley S, Patel M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med 2013;62:121–131.
He S, Yan X. From resveratrol to its derivatives: new sources of natural antioxidant. Curr Med Chem 2013;20:1005–1017.
Scannevin RH, Chollate S, Jung MY, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther 2012;341:274–284.
Shetty AK. Promise of resveratrol for easing status epilepticus and epilepsy. Pharmacol Ther 2011;131:269–286.
Wu Z, Xu Q, Zhang L, et al. Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem Res 2009;34:1393–1400.
Friedman LK, Goldstein B, Rafiuddin A, et al. Lack of resveratrol neuroprotection in developing rats treated with kainic acid. Neuroscience 2013;230:39–49.
Hybertson BM, Gao B, Bose SK, et al. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med 2011;32:234–246
Zhang M, An C, Gao Y, et al. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol 2013;100:30–47.
Winden KD, Karsten SL, Bragin A, et al. A systems level, functional genomics analysis of chronic epilepsy. PLoS One 2011;6:e20763.
Kraft AD, Lee JM, Johnson DA, et al. Neuronal sensitivity to kainic acid is dependent on the Nrf2-mediated actions of the antioxidant response element. J Neurochem 2006; 98:1852–1865.
Mazzuferi M, Kumar G, van Eyll J, et al. Nrf2 defense pathway: Experimental evidence for its protective role in epilepsy. Ann Neurol 2013;74:560–568.
Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci 2013;34:340–346.
Stangel M, Linker RA. Dimethyl fumarate (BG-12) for the treatment of multiple sclerosis. Expert Rev Clin Pharmacol 2013;6:355–362.
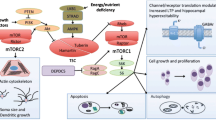
Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol 2005;17:596–603.
Sandsmark DK, Pelletier C, Weber JD, et al. Mammalian target of rapamycin: master regulator of cell growth in the nervous system. Histol Histopathol 2007;22:895–903.
Curatolo P, D'Argenzio L, Cerminara C, et al. Management of epilepsy in tuberous sclerosis complex. Expert Rev Neurother 2008;8:457–467.
Wong M. Mammalian target of rapamycin (mTOR) activation in focal cortical dysplasia and related focal cortical malformations. Exp Neurol 2013;244:22–26.
Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 2009;29:6964–6972.
Canpolat M, Per H, Gumus H, et al. Rapamycin has a beneficial effect on controlling epilepsy in children with tuberous sclerosis complex: results of 7 children from a cohort of 86. Childs Nerv Syst 2014;30:227–240.
McDaniel SS, Wong M. Therapeutic role of mammalian target of rapamycin (mTOR) inhibition in preventing epileptogenesis. Neurosci Lett 2011;497:231–239.
Huang X, Zhang H, Yang J, et al. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis 2010;40:193–199.
Guo D, Zeng L, Brody DL, et al. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One 2013;8:e64078.
Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci 2011;31:2337–2347.
Kumar G, Mazzuferi M, Otoul C, et al. A randomized, blinded preclinical trial of rapamycin in pilocarpine mouse model of chronic epilepsy. Program No. 560.08. 2011 Neuroscience Meeting Planner. Society for Neuroscience, Washington, DC, 2011.
Sliwa A, Plucinska G, Bednarczyk J, et al. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci Lett 2012;509:105–109.
Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med 2002;53:409–435.
Okada K, Yamashita U, Tsuji S. Ameliorative effect of pioglitazone on seizure responses in genetically epilepsy-susceptible EL mice. Brain Res 2006;1102:175–178.
Porta N, Vallée L, Lecointe C, et al. Fenofibrate, a peroxisome proliferator-activated receptor-alpha agonist, exerts anticonvulsive properties. Epilepsia 2009;50:943–948.
Sun H, Huang Y, Yu X, et al. Peroxisome proliferator-activated receptor gamma agonist, rosiglitazone, suppresses CD40 expression and attenuates inflammatory responses after lithium pilocarpine-induced status epilepticus in rats. Int J Dev Neurosci 2008;26:505–515.
Hong S, Xin Y, HaiQin W, et al. The PPARγ agonist rosiglitazone prevents cognitive impairment by inhibiting astrocyte activation and oxidative stress following pilocarpine-induced status epilepticus. Neurol Sci 2012;33:559–566.
Chuang YC, Lin TK, Huang HY, et al. Peroxisome proliferator-activated receptors γ/mitochondrial uncoupling protein 2 signaling protects against seizure-induced neuronal cell death in the hippocampus following experimental status epilepticus. J Neuroinflammation 2012;9:184.
Hong S, Xin Y, HaiQin W, et al. The PPARγ agonist rosiglitazone prevents neuronal loss and attenuates development of spontaneous recurrent seizures through BDNF/TrkB signaling following pilocarpine-induced status epilepticus. Neurochem Int 2013;63:405–412.
Bugge TH, Kombrinck KW, Flick MJ, et al. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell 1996;87:709–719.
Qian Z, Gilbert ME, Colicos MA, et al. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature 1993;361:453–457.
Tsirka SE, Gualandris A, Amaral DG, et al. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue-plasminogen activator. Nature 1995;377:340–344.
Tsirka SE, Bugge TH, Degen JL, et al. Neuronal death in the central nervous system demonstrates a non-fibrin substrate for plasmin. Proc Natl Acad Sci USA 1997;94:9779–9781.
Wu YP, Siao CJ, Lu W, et al. The tissue plasminogen activator (tPA)/plasmin extracellular proteolytic system regulates seizure-induced hippocampal mossy fiber outgrowth through a proteoglycan substrate. J Cell Biol 2000;148:1295–1304.
Takao M, Benson MD, Murrell JR, et al. Neuroserpin mutation S52R causes neuroserpin accumulation in neurons and is associated with progressive myoclonus epilepsy. J Neuropathol Exp Neurol 2000;59:1070–1086.
Yepes M, Sandkvist M, Coleman TA, et al. Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. J Clin Invest 2002;109:1571–1578.
Chateauvieux S, Grigorakaki C, Morceau F, et al. Erythropoietin, erythropoiesis and beyond. Biochem Pharmacol 2011;82:1291–1303.
Mikati MA, El Hokayem JA, El Sabban ME. Effects of a single dose of erythropoietin on subsequent seizure susceptibility in rats exposed to acute hypoxia at P10. Epilepsia 2007;48:175–181.
Bahçekapılı N, Akgün-Dar K, Albeniz I, et al. Erythropoietin pretreatment suppresses seizures and prevents the increase in inflammatory mediators during pentylenetetrazole induced generalized seizures. Int J Neurosci 2014 (in press).
Nadam J, Navarro F, Sanchez P, et al. Neuroprotective effects of erythropoietin in the rat hippocampus after pilocarpine-induced status epilepticus. Neurobiol Dis 2007;25:412–426.
Chu K, Jung KH, Lee ST, et al. Erythropoietin reduces epileptogenic processes following status epilepticus. Epilepsia 2008;49:1723–1732.
Sözmen SÇ, Kurul SH, Yiş U, et al. Neuroprotective effects of recombinant human erythropoietin in the developing brain of rat after lithium-pilocarpine induced status epilepticus. Brain Dev 2012;34:189–195.
Eid T, Brines ML, Cerami A, et al. Increased expression of erythropoietin receptor on blood vessels in the human epileptogenic hippocampus with sclerosis. J Neuropathol Exp Neurol 2004;63:73–83.
Marchi N, Lerner-Natoli M. Cerebrovascular remodeling and epilepsy. Neuroscientist 2013;19:304–312.
Rigau V, Morin M, Rousset MC, et al. Angiogenesis is associated with blood–brain barrier permeability in temporal lobe epilepsy. Brain 2007;130:1942–1956.
Vezzani A. VEGF and seizures: cross-talk between endothelial and neuronal environments. Epilepsy Curr 2005;5:72–74.
McCloskey DP, Croll SD, Scharfman HE. Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J Neurosci 2005;25:8889–8897.
Nicoletti JN, Shah SK, McCloskey DP, et al. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience 2008;151:232–241.
Cammalleri M, Martini D, Ristori C, et al. Vascular endothelial growth factor up-regulation in the mouse hippocampus and its role in the control of epileptiform activity. Eur J Neurosci 2011;33:482–498.
Lampson LA. Monoclonal antibodies in neuro-oncology: Getting past the blood–brain barrier. MAbs 2011;3:153–160.
Croll SD, Goodman JH, Scharfman HE. Vascular endothelial growth factor (VEGF) in seizures: a double-edged sword. Adv Exp Med Biol 2004;548:57–68.
Morin-Brureau M, Rigau V, Lerner-Natoli M. Why and how to target angiogenesis in focal epilepsies. Epilepsia 2012;53(Suppl. 6):64–68.
Lee C, Agoston DV. Inhibition of VEGF receptor 2 increased cell death of dentate hilar neurons after traumatic brain injury. Exp Neurol 2009;220:400–403.
Nikitidou L, Kanter-Schlifke I, Dhondt J, et al. VEGF receptor-2 (Flk-1) overexpression in mice counteracts focal epileptic seizures. PLoS One 2012;7:e40535.
Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med 2001;5:378–387.
Adamson P, Greenwood J. How do statins control neuroinflammation? Inflamm Res 2003;52:399–403.
Lee JK, Won JS, Singh AK, et al. Statin inhibits kainic acid-induced seizure and associated inflammation and hippocampal cell death. Neurosci Lett 2008;440:260–264.
Xie C, Sun J, Qiao W, et al. Administration of simvastatin after kainic acid-induced status epilepticus restrains chronic temporal lobe epilepsy. PLoS One 2011;6:e24966.
Lee CY, Jaw T, Tseng HC, et al. Lovastatin modulates glycogen synthase kinase-3β pathway and inhibits mossy fiber sprouting after pilocarpine-induced status epilepticus. PLoS One 2012;7:e38789.
van Vliet EA, Holtman L, Aronica E, et al. Atorvastatin treatment during epileptogenesis in a rat model for temporal lobe epilepsy. Epilepsia 2011;52:1319–1330.
Osterweil EK, Chuang SC, Chubykin AA, et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron 2013;77:243–250.
Etminan M, Samii A, Brophy JM. Statin use and risk of epilepsy: a nested case–control study. Neurology 2010;75:1496–1500.
McNamara JO, Scharfman HE. Temporal lobe epilepsy and the BDNF receptor, TrkB. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies. 4th ed. National Center for Biotechnology Information, Bethesda, MD, 2012, available at: http://www.ncbi.nlm.nih.gov/books/NBK98186. Accessed March 16, 2014.
Paradiso B, Zucchini S, Su T, et al. Localized overexpression of FGF-2 and BDNF in hippocampus reduces mossy fiber sprouting and spontaneous seizures up to 4 weeks after pilocarpine-induced status epilepticus. Epilepsia 2011;52:572–578.
Bovolenta R, Zucchini S, Paradiso B, et al. Hippocampal FGF-2 and BDNF overexpression attenuates epileptogenesis-associated neuroinflammation and reduces spontaneous recurrent seizures. J Neuroinflammation 2010;7:81.
Liu G, Gu B, He XP, et al. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron 2013;79:31–38.
Weinshenker D, Szot P. The role of catecholamines in seizure susceptibility: new results using genetically engineered mice. Pharmacol Ther 2002;94:213–233.
Pitkänen A, Narkilahti S, Bezvenyuk Z, et al. Atipamezole, an α2-adrenoceptor antagonist, has disease modifying effects on epileptogenesis in rats. Epilepsy Res 2004;61:119–140.
Halonen T, Kotti T, Tuunanen J, et al. Alpha2-adrenoceptor agonist, dexmedetomidine, protects against kainic acid-induced convulsions and neuronal damage. Brain Res 1995;693:217–224.
Wallace MJ, Blair RE, Falenski KW, et al. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther 2003;307:129–137.
Chen K, Neu A, Howard AL, et al. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci 2007;27:46–58.
Echegoyen J, Armstrong C, Morgan RJ, et al. Single application of a CB1 receptor antagonist rapidly following head injury prevents long-term hyperexcitability in a rat model. Epilepsy Res 2009;85:123–127.
Eftekhari S, Mehvari Habibabadi J, Najafi Ziarani M, et al. Bumetanide reduces seizure frequency in patients with temporal lobe epilepsy. Epilepsia 2013;54:9–12.
Löscher W, Puskarjov M, Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology 2013;69:62–74.
Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol 2008;63:222–235.
Mazarati A, Shin D, Sankar R. Bumetanide inhibits rapid kindling in neonatal rats. Epilepsia 2009;50:2117–2122.
Brandt C, Nodadze M, Heuchert N, et al. Disease-modifying effects of phenobarbital and NKCC1 inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. J Neuroscience 2010;30:8602–8612.
Töpfer M, Töllner K, Brandt C, et al. Consequences of inhibition of bumetanide metabolism in rodents on brain penetration and effects of bumetanide in chronic models of epilepsy. Eur J Neurosci 2014;39:673–687.
Engel J Jr, Pitkänen A, Loeb JA, et al. Epilepsy biomarkers. Epilepsia 2013;54(Suppl. 4):61–69.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NS079292) to M.A.R. H.K. and R.M.K. are employees of UCB. M.A.R. declares no competing interests.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaminski, R.M., Rogawski, M.A. & Klitgaard, H. The Potential of Antiseizure Drugs and Agents that Act on Novel Molecular Targets as Antiepileptogenic Treatments. Neurotherapeutics 11, 385–400 (2014). https://doi.org/10.1007/s13311-014-0266-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-014-0266-1