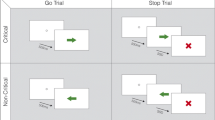
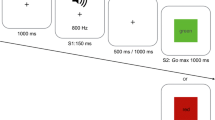
Abstract
Slowness in movement initiation is a cardinal feature of Parkinson’s disease (PD) that is still poorly understood and unsuccessfully alleviated by standard therapies. Here, we raise this major clinical issue within the framework of a novel theoretical model that allows a better understanding of the basic mechanisms involved in movement initiation. This model assumes that movement triggering is inhibited by default to prevent automatic responses to unpredictable events. We investigated to which extent the top-down control necessary to release this locking state before initiating actions is impaired in PD and restored by standard therapies. We used a cue–target reaction time task to test both the ability to initiate fast responses to targets and the ability to refrain from reacting to cues. Fourteen patients with dopaminergic (DA) medication and 11 with subthalamic nucleus (STN) stimulation were tested on and off treatment, and compared with 14 healthy controls. We found evidence that patients withdrawn from treatment have trouble voluntarily releasing proactive inhibitory control; while DA medication broadly reduces movement initiation latency, it does not reinstate a normal pattern of movement initiation; and stimulation of the STN specifically re-establishes the efficiency of the top-down control of proactive inhibition. These results suggest that movement initiation disorders that resist DA medication are due to executive, not motor, dysfunctions. This conclusion is discussed with regard to the role the STN may play as an interface between non-DA executive and DA motor systems in cortico-basal ganglia loops.



Similar content being viewed by others
References
Hallett M. Clinical neurophysiology of akinesia. Rev Neurol (Paris) 1990;146:585-590.
Gauntlett-Gilbert J, Brown VJ. Reaction time deficits and Parkinson's disease. Neurosci Biobehav Rev 1998;22:865-881.
Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bézard E, et al. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128-839.
Jahanshahi M, Brown RG, Marsden CD. The effect of withdrawal of dopaminergic medication on simple and choice reaction time and the use of advance information in Parkinson's disease. J Neurol Neurosurg Psychiatr 1992;55:1168-1176.
Pullman SL, Watts RL, Juncos JL, Chase TN, Sanes JN. Dopaminergic effects on simple and choice reaction time performance in Parkinson's disease. Neurology 1988;38:249-254.
Schubert T, Volkmann J, Müller U, Sturm V, Voges J, Freund H-J, et al. Effects of pallidal deep brain stimulation and levodopa treatment on reaction-time performance in Parkinson's disease. Exp Brain Res 2002;144:8-16.
Limousin P, Martinez-Torres I. Deep brain stimulation for Parkinson's disease. Neurotherapeutics 2008;5:309-319.
Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 2007;318:1309-1312.
Antonelli F, Ray N, Strafella AP. Impulsivity and Parkinson's disease: More than just disinhibition. J Neurol Sci;310:202-207.
Cilia R, Eimeren T. Impulse control disorders in Parkinson’s disease: seeking a roadmap toward a better understanding. Brain Struct Funct 2011;216:289-299.
Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990;13:266-271.
Delong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990;13:281-285.
Haslinger B, Erhard P, Kämpfe N, Boecker H, Rummeny E, Schwaiger M, et al. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain 2001;124:558-570.
Escola L, Michelet T, Macia F, Guehl D, Bioulac B, Burbaud P. Disruption of information processing in the supplementary motor area of the MPTP-treated monkey: a clue to the pathophysiology of akinesia? Brain 2003;126:95-114.
Canavan AG, Nixon PD, Passingham RE. Motor learning in monkeys (Macaca fascicularis) with lesions in motor thalamus. Exp Brain Res 1989;77:113-126.
Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson's disease. Brain 1994;117:877-897.
Ballanger B, Gil R, Audiffren M, Desmurget M. Perceptual factors contribute to akinesia in Parkinson's disease. Exp Brain Res 2007;179:245-253.
Koprich JB, Johnston TH, Huot P, Fox SH, Brotchie JM. New insights into the organization of the basal ganglia. Curr Neurol Neurosci Rep 2009;9:298-304.
Parent A, Levesque M, Parent M. A re-evaluation of the current model of the basal ganglia. Parkinsonism Relat Disord 2001;7:193-198.
Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci 2007;27:11860-11864.
Antonelli F, Ray N, Strafella AP. Imaging cognitive and behavioral symptoms in Parkinson's disease. Exp Rev Neurother 2010;10:1827-1838.
Obeso I, Wilkinson L, Casabona E, Bringas ML, Álvarez M, Álvarez L, et al. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson's disease. Exp Brain Res 2011;212:371-384.
Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, et al. Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson's disease. Brain 2010;133:3611-3624.
Bokura H, Yamaguchi S, Kobayashi S. Event-related potentials for response inhibition in Parkinson's disease. Neuropsychologia 2005;43:967-975.
Gauggel S, Rieger M, Feghoff T-A. Inhibition of ongoing responses in patients with Parkinson's disease. J Neurol Neurosurg Psychiatr 2004;75:539-544.
Falkenstein M, Willemssen R, Hohnsbein J, Hielscher H. Effects of stimulus-response compatibility in Parkinson's disease: a psychophysiological analysis. J Neural Transm 2006;113:1449-1462.
Ballanger B. Top-down control of saccades as part of a generalized model of proactive inhibitory control. J Neurophysiol 2009;102:2578-2580.
Boulinguez P, Jaffard M, Granjon L, Benraiss A. Warning signals induce automatic EMG activations and proactive volitional inhibition: evidence from analysis of error distribution in simple RT. J Neurophysiol 2008;99:1572-1578.
Boulinguez P, Ballanger B, Granjon L, Benraiss A. The paradoxical effect of warning on reaction time: demonstrating proactive response inhibition with event-related potentials. Clin Neurophysiol 2009;120:730-737.
Jaffard M, Benraiss A, Longcamp M, Velay J-L, Boulinguez P. Cueing method biases in visual detection studies. Brain Res 2007;1179:106-118.
Jaffard M, Longcamp M, Velay J-L, Anton J-L, Roth M, Nazarian B, et al. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage 2008;42:1196-1206.
Criaud M, Wardak C, Ben Hamed S, Ballanger B, Boulinguez P. Proactive inhibitory control of response as the default state of executive control. Front Psychol 2012;3:59.
Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends Cogn Sci 2010;14:154-161.
Ballanger B, van Eimeren T, Moro E, Lozano AM, Hamani C, Boulinguez P, et al. Stimulation of the subthalamic nucleus and impulsivity: release your horses. Ann Neurol 2009;66:817-824.
Koerts J, Leenders KL, Brouwer WH. Cognitive dysfunction in non-demented Parkinson's disease patients: Controlled and automatic behavior. Cortex 2009;45:922-929.
van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson's disease? Neuropsychopharmacology 2009;34:2758-2766.
Voon V, Dalley JW. Parkinson disease: impulsive choice-Parkinson disease and dopaminergic therapy. Nat Rev Neurol 2011:7:541-542.
Leh SE, Petrides M, Strafella AP. The Neural circuitry of executive functions in healthy subjects and Parkinson's disease. Neuropsychopharmacology 2009;35:70-85.
Obeso I, Wilkinson L. Levodopa medication does not influence motor inhibition or conflict resolution in a conditional stop-signal task in Parkinson's disease. Exp Brain Res 2011;213:435-445.
Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev 2010;34:50-72.
Eagle DM, Wong JCK, Allan ME, Mar AC, Theobald DE, Robbins TW. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. J Neurosci 2011;31:7349-7356.
Moro E, Esselink RJA, Xie J, Hommel M, Benabid AL, Pollak P. The impact on Parkinson's disease of electrical parameter settings in STN stimulation. Neurology 2002;59:706-713.
Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 2003;349:1925-1934.
Jahanshahi M, Ardouin CM, Brown RG, Rothwell JC, Obeso J, Albanese A, et al. The impact of deep brain stimulation on executive function in Parkinson's disease. Brain 2000;123:1142-1154.
Schroeder U, Kuehler A, Haslinger B, Erhard P, Fogel W, Tronnier VM, et al. Subthalamic nucleus stimulation affects striato-anterior cingulate cortex circuit in a response conflict task: a PET study. Brain 2002;125:1995-2004.
Thobois S, Hotton GR, Pinto S, Wilkinson L, Limousin-Dowsey P, Brooks DJ, et al. STN stimulation alters pallidal-frontal coupling during response selection under competition. J Cereb Blood Flow Metab 2007;27:1173-1184.
Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics 2008;5:294-308.
Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci Res 2002;43:111-117.
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649-2653.
Grande LJ, Crosson B, Heilman KM, Bauer RM, Kilduff P, McGlinchey RE. Visual selective attention in Parkinson's disease: dissociation of exogenous and endogenous inhibition. Neuropsychology 2006;20:370-382.
Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain 1995;118:913-933.
Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 2011;10:734-744.
Jubault T, Monetta L, Strafella AP, Lafontaine A-L, Monchi O. L-dopa medication in Parkinson's disease restores activity in the motor cortico-striatal loop but does not modify the cognitive network. PLoS ONE 2009;4:e6154.
Fimm B, Heber IA, Coenen VA, Fromm C, Noth J, Kronenbuerger M. Deep brain stimulation of the subthalamic nucleus improves intrinsic alertness in Parkinson's disease. Mov Disord. 2009;24:1613-1620.
Jahfari S, Waldorp L, van den Wildenberg WPM, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective Connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci 2011;31:6891-6899.
Zandbelt BB, Bloemendaal M, Neggers SFW, Kahn RS, Vink M. Expectations and violations: Delineating the neural network of proactive inhibitory control. Hum Brain Mapp 2012 Feb 22 [Epub ahead of print].
Marchand WR, Lee JN, Suchy Y, Garn C, Chelune G, Johnson S, et al. Functional architecture of the cortico-basal ganglia circuitry during motor task execution: Correlations of strength of functional connectivity with neuropsychological task performance among female subjects. Hum Brain Mapp 2012 Jan 30 [Epub ahead of print].
Forstmann BU, Anwander A, Schäfer A, Neumann J, Brown S, Wagenmakers E-J, et al. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci USA 2010;107:15916-15920.
Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, et al. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci 2011;14:1462-1467.
Duann J-R, Ide JS, Luo X, Li C-SR. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 2009;29:10171-10179.
Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci 2007;10:240-248.
Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. BPS 2011;69:e55-e68.
Filevich E, Kühn S, Haggard P. Intentional inhibition in human action: the power of 'no'. Neurosci Biobehav Rev 2012;36:1107-1118.
Kühn S, Haggard P, Brass M. Intentional inhibition: how the “veto-area” exerts control. Hum Brain Mapp 2009;30:2834-2843.
Lambert C, Zrinzo L, Nagy Z, Lutti A, Hariz M, Foltynie T, et al. Confirmation of functional zones within the human subthalamic nucleus: Patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage; 2010;60:1-12.
Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, et al. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain 2011;134:359-374.
Temel Y. Subthalamic nucleus stimulation in Parkinson's disease: the other side of the medallion. Exp Neurol 2008;211:321-323.
Benarroch EE. Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology 2008;70:1991-1995.
Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol 1992;32:151-161.
Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, et al. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol 2004;61:1307-1313.
Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage 2011;55:204-215.
Wu T, Long X, Wang L, Hallett M, Zang Y, Li K, et al. Functional connectivity of cortical motor areas in the resting state in Parkinson's disease. Hum Brain Mapp 2011;32:1443-1457.
van Eimeren T, Monchi O, Ballanger B, Strafella AP. Dysfunction of the default mode network in Parkinson Disease: a functional magnetic resonance imaging study. Arch Neurol 2009;66:877-883.
Thiel CM, Fink GR. Visual and auditory alertness: modality-specific and supramodal neural mechanisms and their modulation by nicotine. J Neurophysiol 2007;97:2758-2768.
Klinkenberg I, Sambeth A, Blokland A. Acetylcholine and attention. Behav Brain Res 2011;221:430-442.
Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci 2007;27:3477-3489.
Coull JT, Frith CD, Dolan RJ, Frackowiak RS, Grasby PM. The neural correlates of the noradrenergic modulation of human attention, arousal and learning. Eur J Neurosci 1997;9:589-598.
Coull JT, Büchel C, Friston KJ, Frith CD. Noradrenergically mediated plasticity in a human attentional neuronal network. Neuroimage 1999;10:705-715.
Coull JT, Nobre AC, Frith CD. The noradrenergic alpha2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cereb Cortex 2001;11:73-84.
Witte EA, Marrocco RT. Alteration of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacology 1997;132:315-323.
Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol 1997;11:151-162.
Robbins TW. Chemistry of the mind: Neurochemical modulation of prefrontal cortical function. J Comp Neurol 2005;493:140-146.
Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science 1999;283:549-554.
Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 2005;28:574-582.
Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 1983;9:361-380.
Minzenberg MJ, Yoon JH, Carter CS. Modafinil modulation of the default mode network. Psychopharmacology 2011;215:23-31.
Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res 2011;221:564-573.
Bezard E, Brefel C, Tison F, Peyro-Saint-Paul H, Ladure P, Rascol O, et al. Effect of the alpha 2 adrenoreceptor antagonist, idazoxan, on motor disabilities in MPTP-treated monkey. Prog Neuropsychopharmacol Biol Psychiatry 1999;23:1237-1246.
Delaville C, Deurwaerdère PD, Benazzouz A. Noradrenaline and Parkinson's disease. Front Syst Neurosci 2011;5:31.
Vazey EM, Aston-Jones G. The emerging role of norepinephrine in cognitive dysfunctions of Parkinson's disease. Front Behav Neurosci 2012;6:48.
Acknowledgments
The authors thank the Centre Hospitalier St Jean de Dieu for promoting this research program, and are grateful to Gabrielle Chesnoy for carefully reading and correcting the manuscript.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This work was supported by the Agence Nationale de la Recherche [MNPPS-039-01 to P.B.].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 3215 kb)
Rights and permissions
About this article
Cite this article
Favre, E., Ballanger, B., Thobois, S. et al. Deep Brain Stimulation of the Subthalamic Nucleus, but not Dopaminergic Medication, Improves Proactive Inhibitory Control of Movement Initiation in Parkinson's Disease. Neurotherapeutics 10, 154–167 (2013). https://doi.org/10.1007/s13311-012-0166-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-012-0166-1