Abstract
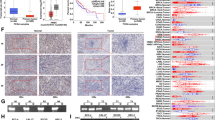
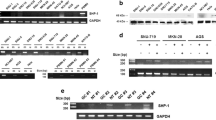
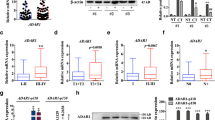
The reversion-inducing cysteine-rich protein with Kazal motifs (RECK) and glycogen synthase kinase (GSK3) are novel tumor suppressors, and emerging evidence has suggested their active role in oral cancer pathogenesis. In the present study, 112 human samples, including 55 fresh samples of 14 adjacent normal tissues, 25 noninvasive oral tumors, and 18 invasive tumors, were included. The messenger RNA (mRNA) expression, protein expression, and promoter methylation of the RECK gene, as well as the expression of GSK3β, phospho/total β-catenin, and c-myc, were measured by RT-PCR, bisulphate modification-PCR, immunohistochemistry, and Western blot analysis. Additionally, ectopic expression of in/active GSK3β was performed in cell culture experiments. This study provided information on the progressive silencing of RECK gene expression at the protein and mRNA levels paralleled with promoter hypermethylation at various stages of oral tumor invasion. RECK expression and the hypermethylation of the RECK gene promoter were negatively and positively correlated with pS9GSK3β/c-myc expression, respectively. Further, a negative trend of RECK protein expression with nuclear β-catenin expression was observed. Induced expression of active GSK3β reversed the RECK silencing in SCC9 cells. Collectively, our results demonstrated that the silencing of the RECK gene, possibly regulated by the GSK3β pathway, is an important event in oral cancer invasion and this pathway could be exploited for therapeutic interventions.






Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50:387–403.
Warnakulasuriya S, Sutherland G, Scully C. Tobacco, oral cancer, and treatment of dependence. Oral Oncol. 2005;41:244–60.
Mahapatra S, Kamath R, Shetty BK, Binu VS. Risk of oral cancer associated with gutka and other tobacco products: a hospital-based case-control study. J Cancer Res Ther. 2015;11:199–203.
Vidya Priyadarsini R, Senthil Murugan R, Nagini S. Aberrant activation of Wnt/beta-catenin signaling pathway contributes to the sequential progression of DMBA-induced HBP carcinomas. Oral Oncol. 2012;48:33–9.
Prgomet Z, Axelsson L, Lindberg P, Andersson T. Migration and invasion of oral squamous carcinoma cells is promoted by WNT5A, a regulator of cancer progression. J Oral Pathol Med. 2015;44:776–84.
Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K, et al. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci U S A. 1998;95:13221–6.
Alexius-Lindgren M, Andersson E, Lindstedt I, Engstrom W. The RECK gene and biological malignancy—its significance in angiogenesis and inhibition of matrix metalloproteinases. Anticancer Res. 2014;34:3867–73.
Yu Y, Hu Y, Li K, Chen Z, Zhang H, Zhang L. RECK gene polymorphism is associated with susceptibility and prognosis of Wilms’ tumor in Chinese children. Med Sci Monit. 2015;21:1928–33.
Span PN, Sweep CG, Manders P, Beex LV, Leppert D, Lindberg RL. Matrix metalloproteinase inhibitor reversion-inducing cysteine-rich protein with Kazal motifs: a prognostic marker for good clinical outcome in human breast carcinoma. Cancer. 2003;97:2710–5.
Namwat N, Puetkasichonpasutha J, Loilome W, Yongvanit P, Techasen A, Puapairoj A, et al. Downregulation of reversion-inducing-cysteine-rich protein with Kazal motifs (RECK) is associated with enhanced expression of matrix metalloproteinases and cholangiocarcinoma metastases. J Gastroenterol. 2011;46:664–75.
Du YY, Dai DQ, Yang Z. Role of RECK methylation in gastric cancer and its clinical significance. World J Gastroenterol. 2010;16:904–8.
Long NK, Kato K, Yamashita T, Makita H, Toida M, Hatakeyama D, et al. Hypermethylation of the RECK gene predicts poor prognosis in oral squamous cell carcinomas. Oral Oncol. 2008;44:1052–8.
Nagini S, Letchoumy PV. A T, Cr R. Of humans and hamsters: a comparative evaluation of carcinogen activation, DNA damage, cell proliferation, apoptosis, invasion, and angiogenesis in oral cancer patients and hamster buccal pouch carcinomas. Oral Oncol. 2009;45:e31–7.
Han L, Yue X, Zhou X, Lan FM, You G, Zhang W, et al. MicroRNA-21 expression is regulated by beta-catenin/STAT3 pathway and promotes glioma cell invasion by direct targeting RECK. CNS Neurosci Ther. 2012;18:573–83.
Lan F, Yue X, Han L, Shi Z, Yang Y, Pu P, et al. Genome-wide identification of TCF7L2/TCF4 target miRNAs reveals a role for miR-21 in Wnt-driven epithelial cancer. Int J Oncol. 2012;40:519–26.
Brenner C, Deplus R, Didelot C, Loriot A, Vire E, De Smet C, et al. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 2005;24:336–46.
Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–76.
Doble BW, Woodgett JR. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs. 2007;185:73–84.
Mishra R, Nagini S, Rana A. Expression and inactivation of glycogen synthase kinase 3 alpha/beta and their association with the expression of cyclin D1 and p53 in oral squamous cell carcinoma progression. Mol Cancer. 2015;14:20.
Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010;9:144.
Zhang C, Ling Y, Zhang C, Xu Y, Gao L, Li R, et al. The silencing of RECK gene is associated with promoter hypermethylation and poor survival in hepatocellular carcinoma. Int J Biol Sci. 2012;8:451–8.
Clark JC, Thomas DM, Choong PF, Dass CR. RECK—a newly discovered inhibitor of metastasis with prognostic significance in multiple forms of cancer. Cancer Metastasis Rev. 2007;26:675–83.
Jung HM, Phillips BL, Patel RS, Cohen DM, Jakymiw A, Kong WW, et al. Keratinization-associated miR-7 and miR-21 regulate tumor suppressor reversion-inducing cysteine-rich protein with kazal motifs (RECK) in oral cancer. J Biol Chem. 2012;287:29261–72.
Hong KJ, Hsu MC, Hou MF, Hung WC. The tumor suppressor RECK interferes with HER-2/neu dimerization and attenuates its oncogenic signaling. FEBS Lett. 2011;585:591–5.
Kitajima S, Miki T, Takegami Y, Kido Y, Noda M, Hara E, et al. Reversion-inducing cysteine-rich protein with Kazal motifs interferes with epidermal growth factor receptor signaling. Oncogene. 2011;30:737–50.
Sasahara RM, Takahashi C, Noda M. Involvement of the Sp1 site in ras-mediated downregulation of the RECK metastasis suppressor gene. Biochem Biophys Res Commun. 1999;264:668–75.
Hsu MC, Chang HC, Hung WC. HER-2/neu represses the metastasis suppressor RECK via ERK and Sp transcription factors to promote cell invasion. J Biol Chem. 2006;281:4718–25.
Liu LT, Peng JP, Chang HC, Hung WC. RECK is a target of Epstein-Barr virus latent membrane protein 1. Oncogene. 2003;22:8263–70.
Mishra R, Das BR. Early overexpression of Cdk4 and possible role of KRF and c-myc in chewing tobacco mediated oral cancer development. Mol Biol Rep. 2003;30:207–13.
Chung TT, Pan MS, Kuo CL, Wong RH, Lin CW, Chen MK, et al. Impact of RECK gene polymorphisms and environmental factors on oral cancer susceptibility and clinicopathologic characteristics in Taiwan. Carcinogenesis. 2011;32:1063–8.
Lin HY, Chiang CH, Hung WC. STAT3 upregulates miR-92a to inhibit RECK expression and to promote invasiveness of lung cancer cells. Br J Cancer. 2013;109:731–8.
Rodriguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011;17:330–9.
Chang HC, Cho CY, Hung WC. Silencing of the metastasis suppressor RECK by RAS oncogene is mediated by DNA methyltransferase 3b-induced promoter methylation. Cancer Res. 2006;66:8413–20.
Kanai Y, Hirohashi S. Alterations of DNA methylation associated with abnormalities of DNA methyltransferases in human cancers during transition from a precancerous to a malignant state. Carcinogenesis. 2007;28:2434–42.
Cardeal LB, Boccardo E, Termini L, Rabachini T, Andreoli MA, di Loreto C, et al. HPV16 oncoproteins induce MMPs/RECK-TIMP-2 imbalance in primary keratinocytes: possible implications in cervical carcinogenesis. PLoS One. 2012;7:e33585.
Mishra R. Biomarkers of oral premalignant epithelial lesions for clinical application. Oral Oncol. 2012;48:578–84.
Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, et al. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14:4814–26.
Packham G, Cleveland JL. c-Myc and apoptosis. Biochim Biophys Acta. 1995;1242:11–28.
Askew DS, Ashmun RA, Simmons BC, Cleveland JL. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–22.
Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–12.
Chang HC, Liu LT, Hung WC. Involvement of histone deacetylation in ras-induced down-regulation of the metastasis suppressor RECK. Cell Signal. 2004;16:675–9.
Mizuno S, Chijiwa T, Okamura T, Akashi K, Fukumaki Y, Niho Y, et al. Expression of DNA methyltransferases DNMT1, 3 A, and 3B in normal hematopoiesis and in acute and chronic myelogenous leukemia. Blood. 2001;97:1172–9.
Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC. TGF-beta1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012;12:26.
Pezeron G, Millen K, Boukhatmi H, Bray S. Notch directly regulates the cell morphogenesis genes Reck, talin and trio in adult muscle progenitors. J Cell Sci. 2014;127:4634–44.
Acknowledgments
The authors wish to acknowledge Prof. M.K. Rai (Pathologist), Director, RIMS, Ranchi, and Prof. NK Jha, Head Dept. of Surgery (and his colleagues) RIMS, Ranchi; and the Director of CARA, Cancer Hospital, Ranchi, and his colleagues Dr. M. Akhouri, Dr.(Md) Aftab A. Ansari, Dr. K. Saurav, and Dr. Raghav Sharan (Clinic), Ranchi, for their cooperation. RM thanks the M.Sc. students (Sunita, Pooja and Meher) and is thankful to Dr. Rupesh Dash, ILS, Bhubaneswar, for his kind support. The fellowship of KKP (JRF-CSIR), AKS (DBT-Research Associate), MA (Project JRF), MA, TK, and PM (CUJ Fellowship) and the financial support from the DBT, New Delhi (Project No. BT/PR4624/MED/30/701/2012; Departmental DBT Builder Programme No. BT/PR9028/INF/22/193/2013), are acknowledged.
Authors’ contributions
KKP, MA, SN, AR, and RM contributed to the IHC, WB, and RT-PCR experiments. KKP, AKS, TK, PM, and RKD contributed to the DNA methylation experiments. KKP, RM, and AR contributed to the cell culture experiments and AKP for statistics. RM has written the MS, and the final version of the MS has been approved by all the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The samples were collected after obtaining informed consent from the patients, and the use of human samples was approved by the Institutional Human Ethical Committee of CUJ. The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Conflicts of interest
None
Role of the funding source
No role except financial assistance.
Rights and permissions
About this article
Cite this article
Pramanik, K.K., Singh, A.K., Alam, M. et al. Reversion-inducing cysteine-rich protein with Kazal motifs and its regulation by glycogen synthase kinase 3 signaling in oral cancer. Tumor Biol. 37, 15253–15264 (2016). https://doi.org/10.1007/s13277-016-5362-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5362-x