Abstract
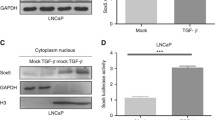
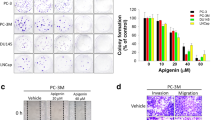
Epithelial-mesenchymal transition (EMT) is a plastic transition in tumor progression during which cancer cells undergo dramatic changes acquiring highly invasive properties. Transforming growth factor-β (TGF-β) is an inducer of EMT in epithelial cells and is obligatory for acquiring invasive phenotype in carcinoma. TGF-β plays a vital role in metastasis and tumorigenesis in prostate cancer, and mutations in the components of Wnt signaling pathways are associated with various kinds of cancers including prostate cancer. The purpose of this study was to identify alterations in Wnt signaling pathway components involved during prostate cancer progression and to determine the effect of quercetin on TGF-β-induced EMT in prostate cancer (PC-3) cell line. The expression of epithelial and mesenchymal markers and the components of Wnt signaling pathway were evaluated by real-time polymerase chain reaction. It was observed that quercetin prevented TGF-β-induced expression of vimentin and N-cadherin and increased the expression of E-cadherin in PC-3 cells, thus preventing TGF-β-induced EMT. Furthermore, the relative expression of Twist, Snail, and Slug showed that quercetin significantly decreased TGF-β-induced expression of Twist, Snail, and Slug. In the present study, the expression of epithelial markers were found to be upregulated in naive state and downregulated in induced state whereas the mesenchymal markers were found to be downregulated in naive state and upregulated in induced state. Thus, our study concludes that quercetin may prevent prostate cancer metastasis by regulating the components of Wnt pathway.






Similar content being viewed by others
References
Siegel R, Miller K, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65(1):29.
Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–29.
Grant CM, Kyprianou N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl Androl Urol. 2013;2(4):202–11.
Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90.
Moustakas A, Heldin P. Biochimica et Biophysica Acta TGF β and matrix-regulated epithelial to mesenchymal transition ☆. BBA - Gen Subj. 2014;1840(8):2621–2634.
Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109(12):1551–9.
Zavadil J, Böttinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–74.
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. Nature Publishing Group2012;13(1):11–26.
Valkenburg KC, Graveel CR, Zylstra-Diegel CR, Zhong Z, Williams BO. Wnt/β-catenin signaling in normal and cancer stem cells. Cancers (Basel). 2011;3(2):2050–79.
Baruah MM, Sharma N. Effect of flavonoid on reversal of TGF-β induced epithelial to mesenchymal transition in prostate cancer (PC-3) cell line. Ind J Clin Biochem. 2015;30(1):S83.
Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets. 2010;9(4):263–85.
Papiez MA, Cierniak A, Krzysciak W, Bzowska M, Taha HM, Jozkowicz A, et al. The changes of antioxidant defense system caused by quercetin administration do not lead to DNA damage and apoptosis in the spleen and bone marrow cells of rats. Food Chem Toxicol. 2008;46(9):3053–8.
Baruah MM, Sharma N, Khandwekar AP, Lavale G-, Author C. Flavonoids and Prostate Cancer. AIJRFANS. 2016;15(1):01–07.
Kim GT, Lee SH, Kim II J, Kim YM. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int J Mol Med. 2014;33(4):863–9.
Chan S-T, Yang N-C, Huang C-S, Liao J-W, Yeh S-L. Quercetin enhances the antitumor activity of trichostatin a through upregulation of p53 protein expression in vitro and in vivo. PLoS One 2013;8(1):e54255.
Shan B-E, Wang M-X, Li R. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer Investig. 2009;27(6):604–12.
Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol. 2004;281:301–11.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–8.
BD Biosciences. Caspase-3 Activation: an indicator of apoptosis in image-based assays. BD Biosciences. 2012. https://www.bdbiosciences.com/documents/Bioimaging_AppNote_Apoptosis.pdf. Accessed 10 Nov 2015.
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51.
Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118(19):4325–6.
Nistico P, Bissell MJ, Radisky DC. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol. 2012;4(2):a011908.
Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–21.
Zhong H, Marzo AM De, Laughner E, Lim M, Hilton D A, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases 1. New York. 2000;(22):5830–5.
Son H, Moon A. Epithelial-mesenchymal transition and cell invasion. Toxicol Res. 2010;26(4):245–52.
Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–69.
López-Nouoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1(6–7):303–14.
Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-β employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174(2):175–83.
Nawshad A, LaGamba D, Polad A, Hay ED. Transforming growth factor-β signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179(1–2):11–23.
Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7443–54.
Malage RD. Studies on regulation of TGF-β and markers of epithelial to mesenchymal transition in breast cancer cells. Dissertation, Jawaharlal Nehru University. 2008;110067.
Chen D, Wilkinson CRM, Watt S, Penkett CJ, Toone WM, Jones N, et al. Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol Biol Cell. 2008;19(1):308–17.
Moustakas A, Heldin C-HH. The regulation of TGFbeta signal transduction. Development. 2009;136(22):3699–714.
Lin YS, Tsai PH, Kandaswami CC, Cheng CH, Ke FC, Lee PP, et al. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells. Cancer Sci. 2011;102(10):1829–39.
Deng XH, Song HY, Zhou YF, Yuan GY, Zheng FJ. Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Exp Ther Med. 2013;6(5):1155–8.
Salem-Alrawaiq N, Abdullah A. A review of flavonoid quercetin: metabolism, bioactivity and antioxidant properties. Int J PharmTech Res. 2014;6(3):933–41.
Uygur B, Wu W-S. SLUG promotes prostate cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol Cancer BioMed Central Ltd; 2011;10(1):139.
Zhang K, Chen D, Jiao X, Zhang S, Liu X, Cao J, et al. Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab Investig. Nature Publishing Group2011;91(3):426–38.
Kurrey NK, Amit K, Bapat SA. Snail and slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97(1):155–65.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis Ben Gurion University of the Negev. Cell. 2004;117:927–39.
Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, et al. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. Elsevier Inc.2014;25(11):1132–9.
Kim H, Seo EM, Sharma AR, Ganbold B, Park J, Sharma G, et al. Regulation of Wnt signaling activity for growth suppression induced by quercetin in 4T1 murine mammary cancer cells. Int J Oncol. 2013;43(4):1319–25.
Novo MCT, Osugui L, dos Reis VO, Longo-Maugeri IM, Mariano M, Popi AF. Blockage of Wnt/β-catenin signaling by quercetin reduces survival and proliferation of B-1 cells in vitro. Immunobiology Elsevier GmbH.; 2015;220(1):60–67.
Maso V, Calgarotto AKA, Franchi GCA, Nowill AED, Filho PLA, Vassallo J, et al. Multitarget effects of quercetin in leukemia. Cancer Prev Res (Phila). 2014;7(12):1240–50.
Zavadil J, Haley J, Kalluri R, Muthuswamy SK, Thompson E. Epithelial-mesenchymal transition. Cancer Res. American Society for Investigative Pathology2008;68(23):9574–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Meghna M. Baruah and Neeti Sharma combined first author.
Electronic supplementary material
Supplementary Table 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Baruah, M.M., Khandwekar, A.P. & Sharma, N. Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumor Biol. 37, 14025–14034 (2016). https://doi.org/10.1007/s13277-016-5277-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5277-6