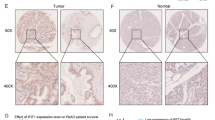
Abstract
Interleukin-22 (IL-22) is an inflammatory cytokine mainly produced by activated Th17 and Th22 cells. The data presented here demonstrate that IL-22 induced the migration and invasion of papillary thyroid cancer (PTC) cells. MicroRNA expression analysis and functional studies indicated that IL-22-mediated migration and invasion is positively regulated by miR-595. Further mechanistic studies revealed that sex-determining region Y-box 17 (Sox17) is directly targeted by miR-595. We then demonstrated that IL-22 regulated migration and invasion of PTC cells via inhibiting Sox17 expression. Interestingly, in PTC cell lines and PTC tissues, expression of IL-22 and miR-595 was upregulated and Sox17 downregulated compared with normal thyroid, and their expression levels were closely correlated. Taken together, this present study suggests that IL-22 stimulation enhances the migration and invasion of PTC cells by regulating miR-595 and its target Sox17.







Similar content being viewed by others
References
Sukumaran R, Kattoor J, Pillai KR, Ramadas PT, Nayak N, Somanathan T, et al. Fine needle aspiration cytology of thyroid lesions and its correlation with histopathology in a series of 248 patients. Indian J Surg Oncol. 2014;5:237–41.
Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74.
Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–80.
Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–93.
Jimenez C, Hu MI, Gagel RF. Management of medullary thyroid carcinoma. Endocrinol Metab Clin North Am. 2008;37:481–96. x-xi.
Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539–47.
Hunt JP, Buchmann LO, Wang L, Abraham D. An analysis of factors predicting lateral cervical nodal metastases in papillary carcinoma of the thyroid. Arch Otolaryngol Head Neck Surg. 2011;137:1141–5.
Onoda N, Ishikawa T, Kawajiri H, Takashima T, Hirakawa K. Pattern of initial metastasis in the cervical lymph node from papillary thyroid carcinoma. Surg Today. 2013;43:178–84.
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–63.
Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67.
Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–71.
Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–63.
Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–32.
Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17:367–80.
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54.
Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23.
Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–15.
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51.
Jiang R, Wang H, Deng L, Hou J, Shi R, Yao M, et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer. 2013;13:59.
Kim K, Kim G, Kim JY, Yun HJ, Lim SC, Choi HS. Interleukin-22 promotes epithelial cell transformation and breast tumorigenesis via MAP3K8 activation. Carcinogenesis. 2014;35:1352–61.
Zhang W, Chen Y, Wei H, Zheng C, Sun R, Zhang J, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14:6432–9.
Petanidis S, Anestakis D, Argyraki M, Hadzopoulou-Cladaras M, Salifoglou A. Differential expression of IL-17, 22 and 23 in the progression of colorectal cancer in patients with K-ras mutation: Ras signal inhibition and crosstalk with GM-CSF and IFN-gamma. PLoS One. 2013;8:e73616.
Xu X, Tang Y, Guo S, Zhang Y, Tian Y, Ni B, et al. Increased intratumoral interleukin 22 levels and frequencies of interleukin 22-producing CD4+ T cells correlate with pancreatic cancer progression. Pancreas. 2014;43:470–7.
Sestito R, Madonna S, Scarponi C, Cianfarani F, Failla CM, Cavani A, et al. STAT3-dependent effects of IL-22 in human keratinocytes are counterregulated by sirtuin 1 through a direct inhibition of STAT3 acetylation. FASEB J. 2011;25:916–27.
Krissansen GW, Yang Y, McQueen FM, Leung E, Peek D, Chan YC, et al. Overexpression of miR-595 and miR-1246 in the sera of patients with active forms of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:520–30.
Chen Y, Wang S, Zhang L, Xie T, Song S, Huang J, et al. Identification of ULK1 as a novel biomarker involved in miR-4487 and miR-595 regulation in neuroblastoma SH-SY5Y cell autophagy. Sci Rep. 2015;5:11035.
Jonatan D, Spence JR, Method AM, Kofron M, Sinagoga K, Haataja L, et al. Sox17 regulates insulin secretion in the normal and pathologic mouse beta cell. PLoS One. 2014;9:e104675.
Chen HL, Chew LJ, Packer RJ, Gallo V. Modulation of the Wnt/beta-catenin pathway in human oligodendroglioma cells by Sox17 regulates proliferation and differentiation. Cancer Lett. 2013;335:361–71.
Yang T, Li XN, Li L, Wu QM, Gao PZ, Wang HL, et al. Sox17 inhibits hepatocellular carcinoma progression by downregulation of KIF14 expression. Tumour Biol. 2014;35:11199–207.
Ye YW, Wu JH, Wang CM, Zhou Y, Du CY, Zheng BQ, et al. Sox17 regulates proliferation and cell cycle during gastric cancer progression. Cancer Lett. 2011;307:124–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All participants gave written informed consent to participate in the study. The study was conducted according to the principles of the Declaration of Helsinki and approved by the Institutional Review Board of the Hubei Cancer Hospital, in accordance with its guidelines for the protection of human subjects.
Financial support
This work was supported by research grants from the Young Scientist Fund of the National Natural Science Foundation of China (81301428) and the Fundamental Research Funds for the Central Universities (2042015kf0188).
The funding agencies had no role in study design, data collection, or analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
None
Additional file
Below is the link to the electronic supplementary material.
ESM 1
Figure S1. IL-22 can not affect the proliferation of these thyroid cancer cells. TPC-1 cells (A) and KAT-5 cells (B) were treated with rhIL-22 for 48 h at the indicated concentrations. The proliferation of the cells were quantified by MTT assay. Bar graphs represent means ± SD, n = 3 (**P < 0.01; *P < 0.05). Figure S2. IL-22 promotes the migration and invasion of KAT-5 cell. Experiments were performed as in Fig 1 except KAT-5 cells were performed. Bar graphs represent means ± SD, n = 3 Supplementary Figure 3. MicroRNA-595 is involved in IL-22 induced migration and invasion of KAT-5 cells. (A) KAT-5 cells were transfected with pre-miR-595 or control for 24 h. Then, cells were treated with or without rhIL-22 (50 ng/ml, 24 h) and allowed to migrate or invade towards serum for 24 h. (B) The experiments were performed as in (A), except the indicated anti-miR-595 or anti-miR control were used. The images shown are representative images from three independent experiments, and a statistical analysis was performed. Bar graphs represent means ± SD, n = 3 (**P < 0.01; *P < 0.05). Figure S4. MiR-595 upregulated the migration and invasion of TPC-1 cells. (A) TPC-1 cells were transfected with pre-miR-595 or control for 48 h at the indicated concentrations. The proliferation of the cells was quantified by MTT assay. (B) Experiments were performed as in A, except the indicated anti-miR-595 or anti-miR control were used. (C, D) TPC-1 cells were transfected with pre-miR-595 or control for 48 h at the indicated concentrations prior to transwell migration (C) or invasion (D) analyses. (E, F) Experiments were performed as in C and D, except the indicated anti-miR-595 or anti-miR control were used. Bar graphs represent means ± SD, n = 3. Figure S5. Sox17 is a target of miR-595. (A, B) KAT-5 cells were co-transfected with either WT or MUT Sox17 3′-UTR reporter plasmids and either pre-miR-595 (A) or anti-miR-595 (B) for 48 h prior to luciferase activity analyses. (C) KAT-5 cells were transfected with pre-miR-595 (left panel) or anti-miR-595 (right panel) for 48 h. Sox17 mRNA was quantified by real-time PCR. Bar graphs represent means ± SD, n = 3 (**P < 0.01; *P < 0.05). Figure S6. The role of Sox17 in the migration and invasion of KAT-5 cells. (A) KAT-5 cells were transfected with pCMV-Sox17 or control vector for 48 h prior to transwell migration and invasion. (B) Experiments were performed as in (A), except cells were transfected with shRNA-control or Sox17 shRNA. The images shown are representative images from three independent experiments, and a statistical analysis was performed. Bar graphs represent means ± SD, n = 3 (**P < 0.01; *P < 0.05). Figure S7. MiR-595 inhibition of KAT-5 cell migration and invasion is mediated by Sox17. Experiments were performed as in Fig 5 except KAT-5 cells were performed. Bar graphs represent means ± SD, n = 3. Table S1: Correlation of IL-22, miR-595 and Sox17 expression with clinicopathologic features in papillary thyroid cancers (PTC). Table S2: Primers Used in Real-time PCR. Table S3: The target sequence of shRNAs. Table S4: Identification of miRNAs differentially expressed in IL-22 treated TPC-1 cells. (DOC 11840 kb)
Rights and permissions
About this article
Cite this article
Mei, Z., Zhou, L., Zhu, Y. et al. Interleukin-22 promotes papillary thyroid cancer cell migration and invasion through microRNA-595/Sox17 axis. Tumor Biol. 37, 11753–11762 (2016). https://doi.org/10.1007/s13277-016-5030-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5030-1