Abstract
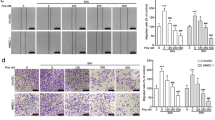
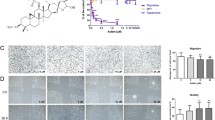
B-9-3, a derivative of harmine, was first synthesized in our laboratory. We have reported that B-9-3 has an anti-proliferative effect against human lung cancer cells via induction of apoptosis and inhibition of cell migration. In the present study, we first studied that the anti-tumor angiogenesis effect and the molecular mechanism of B-9-3-induced tumor vascular degrade and mortify in lung cancer. In vitro, the results showed that B-9-3 selectively inhibited the proliferation of endothelial cells IC50 = 6.16 μg/ml) and vascular fibroblasts (IC50 = 12.59 μg/ml) and induced regression of tumor cells of the following: Lewis lung carcinoma (LLC), Mouse fore-stomach carcinoma (MFC), Human ovarian cancer (SK-OV-3), and prostate cancer (22RV1). Moreover, B-9-3 could significantly increase the apoptosis rate (80.95 %) of vascular endothelial cells, while inhibiting migration of endothelial cells, capillary tube formation of endothelial cells, neovascularization of the rat thoracic aorta ring, and the angiogenesis of chick chorioallantoic membrane (CAM) predominantly through blocking VEGFR2 signaling pathway. In vivo, we investigated the anti-tumor rate and the signal transduction mechanism of B-9-3 by LCC-bearing C57BL/6 mice. The data showed that the tumor inhibition ratio of high dose (20 mg/kg) of B-9-3 was 72.9 %, and quantification of CD34 marker indicated a marked reduction in the number of neovessels after B-9-3 treatment as compared with control group (66.87 %). Remarkably, using IHC and q-RT-PCR, we found that downregulation of the expression of VEGFR2, VEGF-A, and TGFβ1 in tumor confers enhancement to the angiogenesis effect of B-9-3. These data suggest that the angiogenesis inhibitor B-9-3 selectively induces apoptosis of endothelial cells, in part through disruption of VEGF-A/VEGFR2 signaling.







Similar content being viewed by others
References
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249.
Cabebe E, Wakelee H. Role of anti-angiogenesis agents in treating NSCLC: focus on bevacizumab and VEGFR tyrosine kinase inhibitors. Curr Treat Options in Oncol. 2007;8:15.
Zhu Z, Hattori K, Zhang H, Jimenez X, Ludwig DL, Dias S, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia. 2003;17:604.
Doyle B, Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J Am Coll Cardiol. 2007;49:2073.
Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391.
Ling Y, Lu N, Gao Y, Chen Y, Wang S, Yang Y, et al. Endostar induces apoptotic effects in HUVECs through activation of caspase-3 and decrease of Bcl-2. Anticancer Res. 2009;29:411.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335.
Kramer I, Lipp HP. Bevacizumab, a humanized anti-angiogenic monoclonal antibody for the treatment of colorectal cancer. J Clin Pharm Ther. 2007;32:1.
Wells Jr SA, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:134.
Grosios K, Holwell SE, McGown AT, Pettit GR, Bibby MC. In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br J Cancer. 1999;81:1318.
Ferrari G, Pintucci G, Seghezzi G, Hyman K, Galloway AC, Mignatti P. VEGF, a prosurvival factor, acts in concert with TGF-beta1 to induce endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2006;103:17260.
Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol. 2009;219:449.
Kim KY, Lee JW, Ahn BW, Ryu PD, Nam MJ. Loss of endogenous TGF-beta effect induces mouse hepatoma malignancy by correlation with cyclooxygenase-2 and VEGF. Hepatol Res. 2003;26:302.
Malik AK, Baldwin ME, Peale F, Fuh G, Liang WC, Lowman H, et al. Redundant roles of VEGF-B and PlGF during selective VEGF-A blockade in mice. Blood. 2006;107:550.
Pollman MJ, Naumovski L, Gibbons GH. Vascular cell apoptosis: cell type-specific modulation by transforming growth factor-beta1 in endothelial cells versus smooth muscle cells. Circulation. 1999;99:2019.
Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev. 2013;7:199.
S Breza T Jr, Magro CM. Lichenoid and granulomatous dermatitis associated with atypical mycobacterium infections. J Cutan Pathol. 2006;33:512.
Herraiz T, Gonzalez D, Ancin-Azpilicueta C, Aran VJ, Guillen H. beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem Toxicol. 2010;48:839.
Zhong Z, Tao Y, Yang H. Treatment with harmine ameliorates functional impairment and neuronal death following traumatic brain injury. Mol Med Rep. 2015.
Song Y, Wang J, Teng SF, Kesuma D, Deng Y, Duan J, et al. Beta-carbolines as specific inhibitors of cyclin-dependent kinases. Bioorg Med Chem Lett. 2002;12:1129.
Han X, Zhang J, Guo L, Cao R, Li Y, Li N, et al. A series of beta-carboline derivatives inhibit the kinase activity of PLKs. PLoS One. 2012;7:e46546.
Beyer J, Drummer OH, Maurer HH. Analysis of toxic alkaloids in body samples. Forensic Sci Int. 2009;185:1.
Cao R, Peng W, Chen H, Ma Y, Liu X, Hou X, et al. DNA binding properties of 9-substituted harmine derivatives. Biochem Biophys Res Commun. 2005;338:1557.
Piekarski M, Dolhan A, Cielecka-Piontek J, Zalewski P, Kycler W, Kaczmarek A, et al. The influence of pH and temperature on the stability of N-[(piperidine)methylene]daunorubicin hydrochloride and a comparison of the stability of daunorubicin and its four new amidine derivatives in aqueous solutions. Sci World J. 2014;2014:803789.
Phalen DN, Frimberger A, Pyecroft S, Peck S, Harmsen C, Lola S, et al. Vincristine chemotherapy trials and pharmacokinetics in tasmanian devils with tasmanian devil facial tumor disease. PLoS One. 2013;8:e65133.
Daoud A, Song J, Xiao F, Shang J. B-9-3, a novel beta-carboline derivative exhibits anti-cancer activity via induction of apoptosis and inhibition of cell migration in vitro. Eur J Pharmacol. 2014;724:219.
Mousa SA, O’Connor LJ, Bergh JJ, Davis FB, Scanlan TS, Davis PJ. The proangiogenic action of thyroid hormone analogue GC-1 is initiated at an integrin. J Cardiovasc Pharmacol. 2005;46:356.
Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13:1611.
Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE, Rowley DR. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002;62:3298.
Yuan JS, Reed A, Chen F, Stewart Jr CN. Statistical analysis of real-time PCR data. BMC Bioinf. 2006;7:85.
Hamsa TP, Kuttan G. Harmine inhibits tumour specific neo-vessel formation by regulating VEGF, MMP, TIMP and pro-inflammatory mediators both in vivo and in vitro. Eur J Pharmacol. 2010;649:64.
Frost D, Meechoovet B, Wang T, Gately S, Giorgetti M, Shcherbakova I, et al. beta-carboline compounds, including harmine, inhibit DYRK1A and tau phosphorylation at multiple Alzheimer’s disease-related sites. PLoS One. 2011;6:e19264.
Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967.
Liss AS, Thayer SP. Therapeutic targeting of pancreatic stroma. In: Grippo PJ, Munshi HG, editors. Pancreatic cancer and tumor microenvironment. Trivandrum (India). 2012.
Oguro Y, Miyamoto N, Okada K, Takagi T, Iwata H, Awazu Y, et al. Design, synthesis, and evaluation of 5-methyl-4-phenoxy-5H-pyrrolo[3,2-d]pyrimidine derivatives: novel VEGFR2 kinase inhibitors binding to inactive kinase conformation. Bioorg Med Chem. 2010;18:7260.
Zhang J, Li Y, Guo L, Cao R, Zhao P, Jiang W, et al. DH166, a beta-carboline derivative, inhibits the kinase activity of PLK1. Cancer Biol Ther. 2009;8:2374.
Pollman MJ, Naumovski L, Gibbons GH. Endothelial cell apoptosis in capillary network remodeling. J Cell Physiol. 1999;178:359.
Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471.
Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380.
Acknowledgments
This work was supported by grants from the China SME Technology Innovation Fund of Xinjiang Production and Construction Corp (No. 2015AE009)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Qin Ma and Wei Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ma, Q., Chen, W. & Chen, W. Anti-tumor angiogenesis effect of a new compound: B-9-3 through interference with VEGFR2 signaling. Tumor Biol. 37, 6107–6116 (2016). https://doi.org/10.1007/s13277-015-4473-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4473-0