Abstract
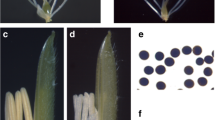
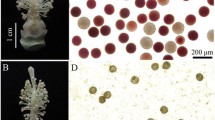
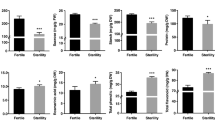
The two-line system has been widely used in hybrid rice seed production, and the reverse photoperiod-sensitive genic male sterile line is a new germplasm with an opposite phenotype compared with normal photoperiod-sensitive genic male sterile rice. To better understand the molecular mechanisms of fertility regulation in reverse photoperiod-sensitive genic male sterile rice, a comparative proteomic approach was used to analyze the protein profiles of three different tissues (young panicles, flag leaves and leaf sheaths) of D52S during the sensitive period of pollen fertility transformation under sterile and fertile conditions. By quantitative analysis, 66 protein spots were identified to be significantly changed in the three tissues. Bioinformatics analyses revealed that in sterile rice, a number of proteins involved in lignin-flavonoid biosynthesis pathway were down-accumulated in panicles. The majority of proteins associated with energy metabolism were down-accumulated in leaf sheaths while the proteins up-accumulated in leaves and leaf sheaths were exclusively photosynthesis and defense related. Based on the proteomics data, a short-day induced male sterility protein network was proposed. In addition, the genes of selected protein spots were further analyzed by qPCR. These findings provide data for better understanding the regulation of pollen fertility in reverse photoperiod-sensitive genic male sterile rice, which could assist in the development of practical reverse photoperiod-sensitive genic male sterile rice for large-scale crop breeding programs.






Similar content being viewed by others
Abbreviations
- β-met:
-
Beta-mercaptoethanol
- LD:
-
Long-day length
- SD:
-
Short-day length
- RPGMS:
-
Reverse photoperiod-sensitive genic male sterile
- EGMS:
-
Environmentally sensitive genetic male sterile
- PGMS:
-
Photoperiod-sensitive genic male sterile
- TGMS:
-
Thermo-sensitive genic male sterile
- NPK:
-
N:P2O5:K2O
References
Agrawal GK, Rakwal R (2011) Rice proteomics: a move toward expanded proteome coverage to comparative and functional proteomics uncovers the mysteries of rice and plant biology. Proteomics 11:1630–1649
Austin MB, Noel JP (2003) The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep 20:79–110
Bevan M, Bancroft I, Bent E, Love K, Goodman H, Dean C, Bergkamp R, Dirkse W, Van Staveren M, Stiekema W (1998) Analysis of 1.9 Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature 391:485–488
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen LY, Xiao YH, Lei DY (2010) Mechanism of sterility and breeding strategies for photoperiod/thermo-sensitive genic male sterile rice. Rice Sci 17:161–167
Chen RZ, Zhao X, Shao Z, Wei Z, Wang YY, Zhu LL, Zhao J, Sun MX, He RF, He GC (2007) Rice UDP-glucose pyrophosphorylase1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell 19:847–861
Chen X, Hu J, Zhang H, Ding Y (2014) DNA methylation changes in photoperiod-thermo-sensitive male sterile rice PA64S under two different conditions. Gene 537:143–148
Cheng LB, Gao XA, Li SY, Shi MJ, Javeed H, Jing XM, Yang GX, He GY (2010) Proteomic analysis of soybean [Glycine max (L.) Meer.] seeds during imbibition at chilling temperature. Mol Breed 26:1–17
Cheng SH, Zhuang JY, Fan YY, Du JH, Cao LY (2007) Progress in research and development on hybrid rice: a super-domesticate in China. Ann Bot 100:959–966
Collman JP, Boulatov R, Sunderland CJ, Fu L (2004) Functional analogues of cytochrome c oxidase, myoglobin, and hemoglobin. Chem Rev 104:561–588
Datta R, Chourey PS, Pring DR, Tang HV (2001) Gene-expression analysis of sucrose-starch metabolism during pollen maturation in cytoplasmic male-sterile and fertile lines of sorghum. Sex Plant Reprod 14:127–134
Denecke J, Goldman M, Demolder J, Seurinck J, Botterman J (1991) The tobacco luminal binding protein is encoded by a multigene family. Plant Cell 3:1025–1035
Ding JH, Lu Q, Ouyang YD, Mao HL, Zhang PB, Yao JL, Xu CG, Li XH, Xiao JH, Zhang QF (2012) A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc Natl Acad Sci 109:2654–2659
Dobritsa AA, Lei ZT, Nishikawa S-i, Urbanczyk-Wochniak E, Huhman DV, Preuss D, Sumner LW (2010) LAP5 and LAP6 encode anther-specific proteins with similarity to chalcone synthase essential for pollen exine development in Arabidopsis. Plant Physiol 153:937–955
Fujii S, Komatsu S, Toriyama K (2007) Retrograde regulation of nuclear gene expression in CW-CMS of rice. Plant Mol Biol 63:405–417
Ge P, Ma C, Wang S, Gao L, Li X, Guo G, Ma W, Yan Y (2012) Comparative proteomic analysis of grain development in two spring wheat varieties under drought stress. Anal Bioanal Chem 402:1297–1313
Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JH, Fisahn J, Höhne M, Günther M, Stitt M (2004) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39:847–862
Goh C-H, Ko S-M, Koh S, Kim Y-J, Bae H-J (2012) Photosynthesis and environments: photoinhibition and repair mechanisms in plants. J Plant Biol 55:93–101
Golding B, Liu X, Li XH, Zhang X, Wang SW (2010) Genetic analysis and mapping of a thermosensitive genic male sterility gene, tms6 (t), in rice (Oryza sativa L.). Genome 53:119–124
Goyer A (2010) Thiamine in plants: aspects of its metabolism and functions. Phytochemistry 71:1615–1624
Grosshans BL, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci 103:11821–11827
Gui JS, Shen JH, Li LG (2011) Functional characterization of evolutionarily divergent 4-coumarate: coenzyme A ligases in rice. Plant Physiol 157:574–586
Guo A, Zheng CX (2013) Female gametophyte development. J Plant Biol 56:345–356
Gutierrez-Carbonell E, Lattanzio G, Sagardoy R, Rodríguez-Celma J, Ríos Ruiz JJ, Matros A, Abadía A, Abadía J, López-Millán AF (2013) Changes induced by zinc toxicity in the 2-DE protein profile of sugar beet roots. J Proteomics 94:149–161
Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin K, Delmer DP (2001) Carbon partitioning to cellulose synthesis. Plant Mol Biol 47:29–51
Hirose T, Scofield GN, Terao T (2008) An expression analysis profile for the entire sucrose synthase gene family in rice. Plant Sci 174:534–543
Hu MQ, Li LQ, Chao JB, Zhao YQ, Zhang ZY, Liang AH (2013) The acidic ribosomal protein P2 from Euplotes octocarinatus is phosphorylated at its N-terminal domain. Biochem Cell Biol 91:1–10
Imin N, Kerim T, Weinman JJ, Rolfe BG (2006) Low temperature treatment at the young microspore stage induces protein changes in rice anthers. Mol Cell Proteomics 5:274–292
Islam N, Lonsdale M, Upadhyaya NM, Higgins TJ, Hirano H, Akhurst R (2004) Protein extraction from mature rice leaves for two-dimensional gel electrophoresis and its application in proteome analysis. Proteomics 4:1903–1908
Joseph CA, Chen Z, Ma D, Zeng HL (2011) Analysis of short photo-periodic sensitive genic male sterility and molecular mapping of rpms3 (t) gene in rice (Oryza sativa L.) using SSR markers. Genes Genom 33:513–519
Kleczkowski LA (1994) Glucose activation and metabolism through UDP-glucose pyrophosphorylase in plants. Phytochemistry 37:1507–1515
Kobayashi T, Kobayashi E, Sato S, Hotta Y, Miyajima N, Tanaka A, Tabata S (1994) Characterization of cDNAs induced in meiotic prophase in lily microsporocytes. DNA Res 1:15–26
Krampitz LO (1969) Catalytic functions of thiamin diphosphate. Annu Rev Biochem 38:213–240
Lang NT, Subudhi PK, Virmani SS, Brar DS, Khush GS, Li ZK, Huang N (1999) Development of PCR-based markers for thermosensitive genetic male sterility gene tms3 (t) in rice (Oryza Sativa L.). Hereditas 131:121–127
LeClere S, Rampey RA, Bartel B (2004) IAR4, a gene required for auxin conjugate sensitivity in Arabidopsis, encodes a pyruvate dehydrogenase E1α homolog. Plant Physiol 135:989–999
Li SL (2004) A study on fertility alteration in yichun in rice D38s with short photoperiod low temperature induced male sterile. J Yichun Univ (Nat Sci) 4:78–81
Li H, Chen Z, Hu M, Wang Z, Hua H, Yin C, Zeng H (2011) Different effects of night versus day high temperature on rice quality and accumulation profiling of rice grain proteins during grain filling. Plant Cell Rep 30:1641–1659
Li JJ, Pandeya D, Jo YD, Liu WY, Kang BC (2013) Reduced activity of ATP synthase in mitochondria causes cytoplasmic male sterility in chili pepper. Planta 237:1097–1109
Li M, Sha A, Zhou X, Yang P (2012) Comparative proteomic analyses reveal the changes of metabolic features in soybean (Glycine max) pistils upon pollination. Sex Plant Reprod 25:281–291
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lu Q, Li XH, Guo D, Xu CG, Zhang Q (2005) Localization of pms3, a gene for photoperiod-sensitive genic male sterility, to a 28.4-kb DNA fragment. Mol Genet Genomics 273:507–511
Ma YJ, Hendershot LM (2004) ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28:51–65
Moffatt BA, Wang L, Allen MS, Stevens YY, Qin W, Snider J, von Schwartzenberg K (2000) Adenosine kinase of Arabidopsis. Kinetic properties and gene expression. Plant Physiol 124:1775–1785
Napoli CA, Fahy D, Wang H, Taylor LP (1999) white anther: A petunia mutant that abolishes pollen flavonol accumulation, induces male sterility, and is complemented by a chalcone synthase transgene. Plant Physiol 120:615–622
Peng HF, Zhang ZF, Wu B, Chen XH, Zhang GQ, Zhang ZM, Wan BH, Lu YP (2008) Molecular mapping of two reverse photoperiod-sensitive genic male sterility genes (rpms1 and rpms2) in rice (Oryza sativa L.). Theor Appl Genet 118:77–83
Pradet-Balade B, Boulmé F, Beug H, Müllner EW, Garcia-Sanz JA (2001) Translation control: bridging the gap between genomics and proteomics? Trends Biochem Sci 26:225–229
Rawlings ND, Barrett AJ (1994) Families of serine peptidases. Method Enzymol 244:19–61
Reflinur Chin JH, Jang SM, Kim B, Lee J, Koh HJ (2012) QTLs for hybrid fertility and their association with female and male sterility in rice. Genes Genom 34:355–365
Samonte SO, Wilson LT, McClung AM, Tarpley L (2001) Seasonal dynamics of nonstructural carbohydrate partitioning in 15 diverse rice genotypes. Crop Sci 41:902–909
Schijlen EG, de Vos CR, Martens S, Jonker HH, Rosin FM, Molthoff JW, Tikunov YM, Angenent GC, van Tunen AJ, Bovy AG (2007) RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol 144:1520–1530
Schluter H, Apweiler R, Holzhutter HG, Jungblut PR (2009) Finding one’s way in proteomics: a protein species nomenclature. Chem Cent J 3:11
Sheoran IS, Saini HS (1996) Drought-induced male sterility in rice: changes in carbohydrate levels and enzyme activities associated with the inhibition of starch accumulation in pollen. Sex Plant Reprod 9:161–169
Shi YL, Zhao S, Yao JL (2009) Premature tapetum degeneration: a major cause of abortive pollen development in photoperiod sensitive genic male sterility in rice. J Integr Plant Biol 51:774–781
Sun QP, Hu CF, Hu J, Li SQ, Zhu YG (2009) Quantitative proteomic analysis of CMS-related changes in Honglian CMS rice anther. Protein J 28:341–348
Tan TW, Lu JK, Nie K, Deng L, Wang F (2010) Biodiesel production with immobilized lipase: a review. Biotechnol Adv 28:628–634
Tschaplinski TJ, Standaert RF, Engle NL, Martin MZ, Sangha AK, Parks JM, Smith JC, Samuel R, Jiang N, Pu Y (2012) Down-regulation of the caffeic acid o-methyltransferase gene in switchgrass reveals a novel monolignol analog. Biotechnol Biofuels 5:1–15
Valledor L, Jorrín J (2011) Back to the basics: maximizing the information obtained by quantitative two dimensional gel electrophoresis analyses by an appropriate experimental design and statistical analyses. J Proteomics 74:1–18
Van Der Meer IM, Stam ME, van Tunen AJ, Mol JN, Stuitje AR (1992) Antisense inhibition of flavonoid biosynthesis in petunia anthers results in male sterility. Plant Cell 4:253–262
Wang B, Xu WW, Wang JZ, Wu W, Zheng HG, Yang ZY, Ray JD, Nguyen HT (1995) Tagging and mapping the thermo-sensitive genic male-sterile gene in rice (Oryza sativa L.) with molecular markers. Theor Appl Genet 91:1111–1114
Wang W, Liu ZW, Guo ZB, Song GY, Cheng Q, Jiang DM, Zhu YG, Yang DC (2011) Comparative transcriptomes profiling of photoperiod-sensitive male sterile rice Nongken 58S during the male sterility transition between short-day and long-day. BMC Genome 12:462
Wang D, Oses-Prieto JA, Li KH, Fernandes JF, Burlingame AL, Walbot V (2010) The male sterile 8 mutation of maize disrupts the temporal progression of the transcriptome and results in the mis-regulation of metabolic functions. Plant J 63:939–951
Wang K, Peng XJ, Ji YX, Yang PF, Zhu YG, Li SQ (2013) Gene, protein, and network of male sterility in rice. Front Plant Sci 4:92
Wang YG, Xing QH, Deng QY, Liang FS, Yuan LP, Weng ML, Wang B (2003) Fine mapping of the rice thermo-sensitive genic male-sterile gene tms5. Theor Appl Genet 107:917–921
Wei MM, Song MZ, Fan SL, Yu SX (2013) Transcriptomic analysis of differentially expressed genes during anther development in genetic male sterile and wild type cotton by digital gene-expression profiling. BMC Genomic 14:97
Weigelt K, Küster H, Rutten T, Fait A, Fernie AR, Miersch O, Wasternack C, Emery RN, Desel C, Hosein F (2009) ADP-glucose pyrophosphorylase-deficient pea embryos reveal specific transcriptional and metabolic changes of carbon-nitrogen metabolism and stress responses. Plant Physiol 149:395–411
Wen L, Liu G, Li SQ, Wan CX, Tao J, Xu KY, Zhang ZJ, Zhu YG (2007) Proteomic analysis of anthers from Honglian cytoplasmic male sterility line rice and its corresponding maintainer and hybrid. Bot Stud 48:293–309
Xiao X, Yang Y, Yang Y, Lin J, Tang D, Liu X (2009) Comparative analysis of young panicle proteome in thermo-sensitive genic male-sterile rice Zhu-1S under sterile and fertile conditions. Biotechnol Lett 31:157–161
Yan JX, Wait R, Berkelman T, Harry RA, Westbrook JA, Wheeler CH, Dunn MJ (2000) A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis 21:3666–3672
Yue J, Ren Y, Wu S, Zhang X, Wang H, Tang C (2014) Differential proteomic studies of the genic male-sterile line and fertile line anthers of upland cotton (Gossypium hirsutum L.). Genes Genom:1–12 doi: 10.1007/s13258-014-0176-y
Zhang H, Xu C, He Y, Zong J, Yang X, Si H, Sun Z, Hu J, Liang W, Zhang D (2013) Mutation in CSA creates a new photoperiod-sensitive genic male sterile line applicable for hybrid rice seed production. Proc Natl Acad Sci 110:76–81
Zhang PB, Ding JH, Zhang QF (2010) The leaves and sites for inducing fertility change by photoperiod in photoperiod-sensitive genic male sterile rice. Mol Plant Breed 8:641–646
Zheng R, Yue SJ, Xu XY, Liu JY, Xu Q, Wang XL, Han L, Yu DY (2012) Proteome analysis of the wild and YX-1 male sterile mutant anthers of wolfberry (Lycium barbarum L.). PLoS ONE 7:e41861
Zhou H, Liu QJ, Li J, Jiang DG, Zhou LY, Wu P, Lu S, Li F, Zhu LY, Liu ZL (2012) Photoperiod-and thermo-sensitive genic male sterility in rice are caused by a point mutation in a novel noncoding RNA that produces a small RNA. Cell Res 22:649–660
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31371600).
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
The experimental design of this study. (TIFF 4,960 kb)
Supplementary Fig. S2
Close-up views of the regions showing the distribution of some differentially accumulated protein spots. In each panel, protein patterns of D52S under SD (left) and LD (middle) conditions are shown. Differentially accumulated protein spots are indicated by black arrows. The quantitative analysis of each protein spot is presented in the right column. The data are the mean values ±SEM (n = 3). (TIFF 3,403 kb)
Supplementary Fig. S3
The category distribution of the identified protein spots from young panicles, leaf sheaths and leaves analysed by WEGO. (TIFF 913 kb)
Rights and permissions
About this article
Cite this article
Chen, Z., Li, H., Ma, X. et al. Proteome alterations of reverse photoperiod-sensitive genic male sterile rice (Oryza sativa L.) at fertility transformation stage. Genes Genom 36, 711–726 (2014). https://doi.org/10.1007/s13258-014-0205-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-014-0205-x