Abstract
Objectives
Although rectal cancer is by far and large the most common pathology involving the rectum that needs imaging, there are many other important but less common pathological conditions affecting anorectal region. The objective of this pictorial review is to discuss the cross-sectional imaging features of less common anorectal and perirectal diseases.
Results
Although a specific histological diagnosis cannot usually be made due to considerable overlap in the imaging appearances of anorectal diseases, this review illustrates the cross-sectional imaging findings with emphasis on magnetic resonance imaging (MRI) that can help in narrowing down the differentials to a reasonable extent.
Teaching points
• Variety of pathology exists in the anorectum apart from common rectal carcinoma
• Anorectal diseases present as non-specific wall thickening indistinguishable from rectal carcinoma
• Computed tomography (CT) and MRI can help in narrowing down the differentials, although often biopsy is warranted.
Similar content being viewed by others
Introduction
Primary rectal adenocarcinoma is a common malignancy with a high mortality rate in the western world. Initially computed tomography (CT) and endoluminal ultrasound (EUS) have been the mainstay of diagnosis and staging. In the past decade magnetic resonance imaging (MRI) has become the imaging modality of choice for loco-regional staging of rectal cancer. The complex anatomy of the anorectal region makes imaging and interpretation challenging. The advent of MRI, with its high soft tissue contrast, multiplanar capability and no radiation risk, has simplified imaging of the anorectal region. The role of MRI in preoperative staging of rectal cancer has been well established now. Though rectal cancer is the commonest cause of rectal mass, there are many other common and uncommon diseases which affect rectum and perirectal region, many of which can mimic rectal carcinoma and their distinction is essential as the management strategy changes significantly [1]. The objective of this review is to describe and illustrate the specific imaging findings of uncommon and atypical diseases affecting the anorectal region. Although a specific diagnosis cannot usually be made due to considerable overlap in the imaging appearances of anorectal diseases, this review illustrates the cross-sectional imaging findings with emphasis on MRI that can help to narrow down the differential diagnoses to a reasonable extent. Uncommon anorectal diseases include congenital cysts, benign and malignant neoplasms excluding adenocarcinoma, atypical infections, inflammatory conditions (excluding common inflammatory bowel disease and post radiation changes) and a few other rare miscellaneous conditions.
MR imaging technique
MRI of the rectum may be performed with either an endorectal coil or a phased-array surface coil. The decision depends on the availability of coils, technical expertise of radiologists, surgeon’s preference and practical issues like time constraints. In our institution we use phased array surface coils routinely for all rectal diseases. The advantage of an endorectal coil lies in its high-resolution images that fully depict the wall layers of the bowel but has the disadvantage in evaluating rectal strictures, high rectal carcinomas and assessment of the perirectal structures due to its smaller field of view (FOV). Phased-array surface coil yields high-spatial-resolution images, albeit with less distinction of bowel wall layers but with additional advantage of a large field of view, patient comfort and ease of use in structuring cancers and high rectal, rectosigmoid tumours [2, 3].
In our institution we do no routine bowel preparation, rectal contrast or antispasmodic agents. Opacification rectal lumen with contrast is still controversial with use of various agents like super paramagnetic iron oxide solutions, methylcellulose, barium suspensions and aqueous gel [4].
A sagittal T2-weighted turbo spin-echo (TSE) sequence is obtained first to locate the rectal lesion. Based on the sagittal sequence, axial and coronal T2-weighted TSE sequences are planned, and they are angled to the plane exactly perpendicular and parallel to the lesion. We have a standard rectal cancer protocol which is applied for all other rectal lesions as well [2]. Our MRI protocol consists of sagittal T2-weighted single-shot images and T2-weighted TSE images in the axial and coronal planes. High-resolution images are obtained in the axial and coronal planes with a slice thickness of 3 mm and small FOV. Unenhanced and contrast-enhanced axial and coronal high-resolution T1-weighted fat-saturated images of the rectum are also obtained. The use of gadolinium in rectal cancers is now becoming optional, as all the staging parameters are adequately depicted in T2-weighted sequences. However, rectal lesions other than usual cancers still benefit from gadolinium. As of now, we routinely do post gadolinium multiplanar T1 VIBE (volume interpolated breath-hold examination) sequences.
Diffusion-weighted imaging (DWI) is a functional imaging tool that yields information about water mobility and tissue cellularity. It also allows calculation of the apparent diffusion coefficient (ADC) from images with different b values. Although diffusion sequences are not part of our routine rectal MRI protocol, there is increasing literature on their usefulness, especially for malignant lesions. The reader is requested to refer to various articles available on principles, imaging parameters and pitfalls of diffusion imaging, as these are beyond the scope of our article [5, 6]. Malignant tumours are generally depicted as foci of increased intensity on DWI and decreased signal intensity on ADC images, because water diffusion is restricted in highly cellular tissues in malignant tumours [5, 7, 8]. However, blood, fat, abscesses, lymph nodes, and melanin can show restricted diffusion and can be resolved by referring to standard T1- and T2-weighted images [9]. Examples include dermoid cyst, rectal lipoma, melanoma and endometriosis. DWI has been proven useful in diagnosis, assessing treatment response and recurrence of rectal carcinoma [10–12]. Other malignant lesions that can show diffusion restriction include lymphoma, stromal tumours and mucinous carcinoma [10, 13–15]. Data on other rectal tumours are not well established and mostly are extrapolated from similar tumours occurring at other sites. Cystic lesions (duplication cyst, tail gut cyst, pseudomyxoma) show T2 shine-through effect, which is high signal intensity on low- and high-b-value images and on ADC images [9].
Developmental retro-rectal cysts
Developmental cysts are benign epithelial cysts in the retro-rectal space, arising from caudal embryonic vestiges. Often these are incidentally detected in middle-aged women. Developmentally these are sub-typed in to epidermoid cysts, dermoid cysts, enteric cysts and neuroenteric cysts. These cysts, when large, can create a mass effect on the rectum and can indent the rectum mimicking sub-mucosal mass.
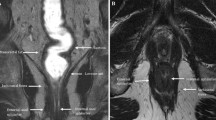
Enteric cysts are most frequent among these and are lined with enteric mucosa. It includes retro-rectal cystic hamartoma (RRCH), also known as tail gut cyst and rectal duplication cyst. Enteric cysts typically are unilocular or multilocular lesions that are hypointense on T1 and hyperintense on T2 without internal enhancement (Fig. 1). RRCH is often multiloculated and may have a small peripheral cyst with honeycomb appearance, which is an important differentiating feature from the other types of developmental cysts. Rectal duplication cysts are different from RRCH in that they have muscular layer and the only lesion in contiguity or continuity with the rectum. They can be associated with genitourinary anomalies.
Neuroenteric cysts are differentiated from other cysts only on histology and are indistinguishable on imaging. Epidermoid and dermoid cysts are lined with stratified squamous epithelium and they have classical features as in any other body location. Epidermoid cysts are unilocular with thin wall and clear fluid. Tiny discrete and linear T2 hypointense areas suggesting keratin and diffusion restriction is characteristic of epidermoid cyst [16, 17]. Dermoid cysts contain fat with skin appendages, such as tooth buds, sweat glands and hair follicles. Fat suppression MRI is helpful in confirming the dermoid cyst and can show diffusion restriction [9].
Role of MRI is to locate the cysts in relation to the rectal wall, its continuity with rectal lumen and assess complications [18, 19]. Complications of developmental cysts include infection, haemorrhage, fistula formation and malignant degeneration. High signal on T1-weighted images can be due to mucus, proteinaceous material, haemorrhage or fat. Low-signal areas on T2-weighted images can be due to haemorrhage and aggregates of keratin. Mural nodularity, asymmetric wall thickening and enhancement suggest malignant change.
Neoplasms
Primary adenocarcinoma is the commonest rectal neoplasm, constituting 90–95 % of rectal cancers. Other less common tumours include benign neoplasms like lipoma, leiomyoma, schwannoma, villous adenoma, cavernous haemangioma and malignant neoplasms like lymphoma, gastrointestinal stromal tumours (GIST), carcinoid, melanoma, Kaposi sarcoma and secondary neoplastic involvement [3].
Benign neoplasms
Lipoma
Rectal lipoma is an uncommon benign submucosal tumour arising from deposits of adipose connective tissue in the bowel wall. Almost 70 % of gastrointestinal lipomas are located in the right colon, and their frequency gradually decreases from cecum to rectum [20]. The most common symptoms are abdominal pain, bleeding, signs of obstruction, signs of intussusception.
Most lipoma present as a broad-based mass with a smooth, well-demarcated margin. Although submucosal in origin, lipoma occasionally evolves into pedunculated lesions. CT shows typical fat attenuation mass in the bowel wall [21]. MRI readily demonstrates the fatty nature of the mass as being isointense compared with the subcutaneous fat on T1-weighted imaging, and low signal intensity on T1-weighted imaging with fat suppression, which confirms the diagnosis of lipoma. Contrast enhancement is variable, usually mild [22].
Leiomyoma
Leiomyoma is a benign smooth muscle tumour and differs clinically, histologically and immunophenotypically from gastrointestinal stromal tumours (GISTs). Leiomyoma shows positivity for smooth muscle actin and desmin, and they are not kit-positive in contrast to that of GISTs. Exact incidence is not known as only few scattered case reports are available. It rarely occurs in the rectum. Appearance is non-specific on CT with localised intramural soft tissue mass showing mild to variable contrast enhancement. Cross-sectional imaging may demonstrate an exoenteric component in some cases. The MRI findings of rectal leiomyomas have not been well described, but it is reported that they are isointense to mildly hyperintense on T2-weighted images when compared with that of muscle [1].
Villous adenoma
Adenomas of the colon are divided into three subtypes: tubular, villous and tubulo-villous. Villous adenoma constitutes approximately 10 % of all adenomas of the colon and is frequently encountered in the rectum and the sigmoid of elderly patients. Malignant potential of adenomatous polyps increases with size, villous configuration and degree of dysplasia. Patient often present with rectal bleeding, electrolyte imbalance (secrete copious amounts of mucoid material rich in protein and potassium) or difficulty in defecation. On imaging villous adenoma are frequently large with flat carpet like or cauliflower/frond like appearance (Figs. 2 and 3). Central vascular stalk with secondary branches is characteristic [23]. Surgical treatment is indicated as villous tumours have a 10–40 % chance of malignant transformation.
A 61 year-old-man presenting to the emergency department with severe dehydration resulting from intermittent episodes of diarrhoea and profuse mucous discharge from the rectum, found to have villous adenoma. a Sagittal T2-weighted MRI and b axial T2-weighted MRI reveal a carpet-like “hairy” lesion covering the mucosa of the rectum (arrows). The lumen of the rectum is distended and fluid filled (arrowhead) with no proximal obstruction. c Contrast-enhanced MRI reveals enhancement of the thickened abnormal mucosa (arrows)
A 65-year-old woman presenting with intermittent diarrhoea, rectal bleeding and difficulty in defecation for 1 month. Laboratory data revealed hypokalaemia. a Coronal contrast CT reveals a lobulated solid mass (arrow) projecting into the lumen of the rectum. b Coronal T2-weighted MRI reveals the frond-like morphology of the lesion (arrow). c Coronal contrast MRI depicts a central vascular stalk (arrow) and the cauliflower or frond-like morphology (arrowhead), classical for villous adenoma
Schwannoma
Rectal schwannomas are uncommon, the common sites in the gastrointestinal tract being oesophagus, stomach and small intestine in that order. Imaging findings are indistinguishable from other benign submucosal tumours like GIST and leiomyoma. On cross-sectional imaging they appear as encapsulated homogenous masses showing mild contrast enhancement (Fig. 4) [24]. Benign schwannomas were often misdiagnosed as leiomyomas, or leiomyosarcomas without immunohistochemical studies.
Diffuse cavernous haemangioma
Rare benign vascular malformation or hamartoma and is not a true neoplasm. The classical clinical triad of hematochezia, cutaneous haemangioma and ectopic pelvic phleboliths on radiograph should make one suspicious of intestinal haemangioma before the advent of cross-sectional imaging. Markedly thickened rectosigmoid wall showing moderately high T2-weighted signal intensity with high signal heterogeneous perirectal tissue with enhancing serpiginious structures (small vessels supplying the malformation) are the characteristic MRI findings (Fig. 5) [25, 26]. MRI may also help in identifying the extent of involvement and for possible extension in to bladder or even sacrum.
Diffuse cavernous haemangioma of the rectum in a 21-year-old man presenting with painless rectal bleeding. a Coronal and b axial fat-suppressed contrast-enhanced T1-weighted MRI reveal enhancing concentric rectal wall thickening (arrows) with enhancing serpiginious structures (arrowhead) in the mesorectum
Malignant neoplasms
Uncommon or atypical rectal carcinoma
Mucinous (colloid) and signet ring are two uncommon histological subtypes of rectal cancer with different biological behaviour and imaging appearances. Mucinous or colloid carcinoma is characterised by the production of an abundant amount of extracellular mucin. It has greater tendency for metastases and local recurrence with 5-year survival rate of 11 % compared with 57 % for non-mucinous carcinomas. Typical MRI features include high signal intensity on T2-weighted images with higher tumour-to-muscle, tumour-to-fat and tumour-to-urine signal intensity ratios. The solid component of the tumour can show diffusion restriction. Contrast-enhancement pattern is peripheral or heterogeneous with lace-like peripheral enhancement being characteristic (Fig. 6) [27].
Mucinous (colloid) carcinoma of the rectum. a Axial and b sagittal T2-weighted MRI show intensely hyperintense heterogeneous rectal mass infiltrating the adjacent structures (T4 stage). (c) Sagittal fat-suppressed contrast-enhanced T1-weighted MRI reveal mesh-like peripheral enhancement of the rectal mass
Signet ring carcinoma is a rare type of rectal carcinoma, similar to the mucinous type in having a mucinous component, but with more aggressive behaviour in the form of a more advanced stage of initial diagnosis, more lymphatic and local spread and poor response to surgery. The most common imaging features are long segment of concentric wall thickening and a target appearance similar to those of metastatic linitis plastica consisting of thickened inner (mucosa and submucosa) and outer (serosa) layers and a relatively thin hypoattenuated middle layer (muscularis propria), or bowel wall thickening with homogeneous attenuation [28–30].
Lymphoma
Primary rectal lymphoma constitutes only 0.1 % of rectal tumours. The diagnosis of primary rectal lymphomas requires that there is a lymphoproliferative disorder confined to the rectum and regional lymph nodes without involvement of abdominal organs, bone marrow and without retroperitoneal or mediastinal lymphadenopathy. Risk factors include AIDS, inflammatory bowel disease and post-transplant status. Rectal lymphoma can present as intraluminal polypoidal mass or diffuse concentric wall thickening. Aneurismal dilatation with minimal or no obstruction is typical with occasional excavating mass with or without fistulisation [31]. It is usually homogenous with intermediate signal on T1 and high signal on T2 with mild to moderate enhancement (Fig. 7). Few recent studies have shown the potential of whole-body DWI in lymphoma for nodal staging, extranodal spread and response assessment. Lymphomatous tissue shows high signal on DWI and low ADC [14]. Differentiating features from adenocarcinoma include preservation of the extramural fat planes, luminal restriction without significant obstruction and thickening of adjacent levator ani [22]. However, it is indistinguishable in a significant number of patients and biopsy is needed for definite diagnosis as the treatment strategies differ considerably. One rare subtype, mantle cell lymphoma commonly presents as multiple polyps called lymphomatous polyposis and can mimic rectal adenomatous polyposis [32] (Fig. 8).
Burkitt cell lymphoma of the rectum in a 16-year-old male. a Axial CT shows Bulky tumour (arrow) infiltrating the rectum with luminal compromise. b Axial and c sagittal T2-weighted MRI reveal infiltration of the submucosal and muscular layers of the rectum with the tumour (arrow). The mucosal architecture and the shape of the rectum are maintained. The tumour is homogeneously isointense to hypointense on T2-weighted MRI. There is extensive perirectal infiltration (arrowhead). d Axial T1-weighted MRI reveals the isointense tumour (arrow) and e contrast-enhanced MRI reveals mild enhancement (arrow)
Mantle cell lymphoma in a 58-year-old man presenting with rectal bleeding and tenesmus.a Coronal, b axial and c sagittal contrast-enhanced CT reveal a long segment of marked circumferential wall thickening (arrow) involving the rectum with multiple polypoidal intraluminal projections. There is aneurismal dilatation of the rectum with extensive retroperitoneal lymphadenopathy (arrowhead)
Stromal tumours
Gastrointestinal stromal tumours are characterised by unique expression of the c-kit receptor (CD117, tyrosine growth factor receptor), which makes them targets for kit inhibitor therapy. The stomach (60–70 %) and small intestine (20–25 %) are the commonest sites, with the rectum involved in 5–7 % of cases. It is common in males over 50 years. MRI features include well-defined eccentric submucosal masses with an exophytic component showing low signal intensity on T1-weighted imaging, isointense to hyperintense on T2-weighted imaging with marked heterogeneous enhancement. T1 hyperintense areas may suggest haemorrhage and T2 hyperintensity suggest cystic change (Figs. 9 and 10). Large necrotic tumours may cavitate and contain air due to communication with the rectal lumen [33, 34]. The most common mode of spread is peritoneal and haematogenous dissemination to the liver; lymph nodal spread is uncommon. Absence of perirectal adenopathy, despite the large size of the tumour, may suggest GIST instead of adenocarcinoma [35]. Recently DWI has been shown to be potentially capable of offering similar information as PET/CT for diagnosis and treatment response as GISTs show diffusion restriction [15].
Rectal GIST in a 62-year-old man presenting with pain and rectal bleeding. a Sagittal T2-weighted MRI reveals a heterogeneous isointense to hypointense mass (arrow) arising from the anterior wall of the rectum and projecting into the rectoprostatic space. b Sagittal T1-weighted MRI reveals the lesion to be isointense on T1-weighted MRI with a few areas of hyperintensity (arrowheads) representing haemorrhage. c Sagittal and d axial contrast-enhanced MRI demonstrates intense enhancement (arrow) with central areas of non-enhancement (arrowhead) representing necrosis
Rectal GIST in a 55-year-old man with a palpable mass on digital rectal examination. a Transrectal ultrasound reveals a hypoechoic vascular mass (arrow) arising from the rectal wall. b Sagittal and c axial T2-weighted MRI reveals the lesion (arrow) to be heterogeneously hypointense and is arising exophytically from the right anterolateral wall. d Sagittal fat-suppressed T1-weighted MRI reveals the lesion (arrow) to be intermediate signal intensity and e contrast-enhanced T1-weighted MRI reveals intense enhancement (arrow). f The mass is bright on diffusion-weighted (b-1,000) MRI
Neuroendocrine tumours
Neuroendocrine tumours (NET) are categorised into three groups: well-differentiated neuroendocrine tumour (benign behaviour or uncertain malignant potential; synonymous with carcinoid in gastro-enteric NETs), well-differentiated neuroendocrine carcinoma (low-grade malignancy; synonymous with malignant carcinoid) and poorly differentiated neuroendocrine carcinomas (high-grade malignancy; usually small cell neuroendocrine carcinomas) [36]. Rectum is the second most common gastrointestinal tract carcinoid following small bowel and comprises 27.4 % of all carcinoid of GI tract [37]. Rectal carcinoid is a submucosal tumour with a more indolent course compared with adenocarcinoma. Metastasis occurs in 4–18 % of cases [38]; most commonly to bone, liver and lymph nodes and has better prognosis in rectum compared with other sites. It is located commonly in the mid-rectum on the anterior and lateral wall [39]. On MRI they appear as a solitary, small, submucosal, polypoidal mass, isointense on T1 and isointense to hyperintense on T2 with marked homogenous enhancement [40] (Fig. 11). Management decisions are based on tumour size, necrosis, depth of invasion, mitosis and angiolymphatic invasion. Current treatment recommendations are: <1 cm—endoscopic or trans-anal resection; 1–2 cm, without muscular invasion or lymph node metastasis—wide excision; 2 cm or greater, muscular invasion or lymph node metastasis—radical surgery [22].
Anorectal melanoma
Primary melanoma of anorectum is a rare tumour accounting for 0.2–0.3 % of rectal cancers. Typical imaging findings include polypoid intraluminal fungating mass expanding the rectum without obstruction. Bulky perirectal lymphadenopathy is almost always seen (Fig. 12). High T1 signal due to melanin is characteristic but variable as 10–30 % of melanomas are amelanotic type [22]. Sixty percent have already metastasis at the time of initial diagnosis, which includes lymph node, cutaneous and visceral secondaries.
Primary amelanotic anorectal melanoma. a, b Axial T2-weighted MRI reveals fungating intraluminal mass (arrow) with a large perirectal deposit (arrowhead). c Sagittal T2-weighted MRI demonstrates the polypoidal mass (arrow) expanding the rectum and the large perirectal deposit (arrowhead). d Axial T1-weighted post contrast MRI reveals heterogeneous enhancement of the mass (arrow) and the deposit (arrowhead)
Kaposi sarcoma
Kaposi sarcoma is a low-grade mesenchymal tumour that involves the blood vessels and lymphatics. It primarily affects skin and disseminates in multiple organs usually seen in AIDS or other immunosuppressive states. Digestive tract is involved in more than 50 % of patients, the rectum being second most commonly involved after the duodenum. Commonly present as polypoidal submucosal masses or irregular fold thickening with hyperattenuating disseminated lymphadenopathy in 80 % of patients (Fig. 13) [41]. Bleeding is a dreaded complication and may warrant angiography for diagnosis and treatment.
Secondary neoplastic involvement
Secondary neoplastic involvement of rectum is uncommon and it is usually by direct invasion from adjacent organs such as the prostate, urinary bladder, uterus and vagina. Prostatic carcinoma, though, grows close to the anterior wall of the rectum, uncommonly involving the rectum as Denonvillier’s fascia is very firm (Fig. 14) [42]. Rectal metastasis is rare and may present as submucosal mass or as linitis plastica of the rectum. Stomach, breast or prostate primaries can result in linitis plastica of the rectum [43]. Typical imaging features are non-distensible rectum with long segment circumferential thickening and narrowing (Fig. 15). It classically shows a target sign on CT and concentric ring pattern on T2-weighted MRI due to exaggeration of normal zonal anatomy by infiltrative tumour in the submucosa and around the muscle layer [30].
Miscellaneous lesions
Deeply infiltrating endometriosis (DIE)
Deeply infiltrating endometriosis is characterised by fibro-muscular hyperplasia that surrounds sparse ectopic endometrial glands. The rectosigmoid is the most common segment of bowel involved. Implants are usually serosal but can erode through subserosal layers and cause thickening and fibrosis of muscularis propria with development of adhesions, bowel strictures and gastrointestinal obstruction. Implants appear as irregular thickening of anterior rectal wall or extramural rectal mass with spiculation and retraction frequently extending to uterosacral ligaments [44, 45, 46]. MRI features are characteristic and include intermediate to high signal on T1 due to blood products and hypointense on T2 due to shading. Shading represents gradual variation in T2 signal due to iron and proteinaceous products from recurrent haemorrhage [47]. Hyperintense foci on T2 may represent ectopic endometrial glands (Fig. 16). However, the disease can also present as fibrous masses that appear to show spiculation and retraction, and hypointense signals on both T1 and T2. Enhancement is variable depending on proportions of inflammatory, glandular and fibrotic tissue [44].
Rectal endometriosis. a Sagittal T2-weighted MRI reveals a hypointense spiculated irregular mass (arrow) along the anterior surface of the mid rectum. b Sagittal T1-weighted MRI reveals this fibrous deposit to be intermediate signal intensity. c Sagittal fat-suppressed T1-weighted MRI demonstrates tiny hyperintense foci (arrowhead) within the endometriotic deposit (arrow). d Axial T2-weighted MRI reveals the irregular spiculated hypointense lesion (arrow) with a few tiny hyperintense foci
Lymphogranuloma venerum (LGV)
A rare sexually transmitted infection seen in homosexual men, caused by Chlamydia trachomatis. It causes ulcerative proctitis mimicking inflammatory bowel disease. Chronic disease leads to strictures and fistulas. Definitive diagnosis is by biopsy and identification of the organism on microbiology [1]. Imaging features are non-specific and include non-specific smooth circumferential thickening of the rectum with adjacent inflammatory stranding. Mucosa is preserved unlike carcinoma (Fig. 17).
Lymphogranuloma venerum in a 26-year-old HIV-positive man. a Axial CT colonography demonstrates diffuse circumferential thickening of the rectum (arrow) with inflammatory stranding in the mesorectum (arrowhead). b Reformatted virtual colonoscopy image demonstrates a smooth stricture (arrow) with maintained mucosa. c Sagittal T2-weighted MRI and d Sagittal contrast-enhanced T1-weighted MRI reveal circumferential thickening (arrow) with luminal compromise of the rectum which is hypointense on T2-weighted MRI and shows enhancement post gadolinium administration. Images in inset are the corresponding axial MR images showing similar findings
Stercoral colitis
Inflammatory colitis related to increased intraluminal pressure from impacted faecal material in the colon. Three most common locations are anterior rectum just proximal to the peritoneal reflection, antimesenteric border of the rectosigmoid and apex of the sigmoid colon [48]. Imaging findings are diagnostic and include faecal impaction with distended affected segment with or without proximal dilatation. Uncomplicated faecal impaction shows a thin wall, whereas focal wall thickening and pericolonic stranding indicate oedema/acute inflammation of stercoral colitis (Fig. 18). Dense mucosa from intramural haemorrhage indicate ischaemia and progresses to mucosal sloughing with perfusion defect indicating infarction [49]. Extra-luminal air and faecalomas protruding through a perforation site indicate perforation. Perforation rent is usually on the antimesenteric border and >1 cm in diameter [50].
Pseudomyxoma retroperitonei
Sub-peritoneal pelvic adenomucinosis (pseudomyxoma retroperitonei) is an extra-peritoneal accumulation of mucin, usually following surgery for mucinous appendiceal neoplasms. These are usually indolent and slow growing, and are lined with glandular epithelium and filled with thick, gelatinous material. CT and MR imaging show well-marginated, homogeneous, septated cystic lesions with mural nodules and calcifications (Fig. 19) [51, 52].
Sub peritoneal pelvic adenomucinosis presenting as slow growing pelvic mass 6 years after surgery for appendiceal mucocele. a Axial contrast CT reveals retrorectal multiloculated cystic lesion (arrow) with calcification (arrowhead). b Axial T2-weighted MRI and c sagittal T2-weighted MRI reveal a cystic lesion (arrow) with multiple septations. d Axial contrast T1-weighted MRI reveals peripheral and septal enhancement of the lesion (arrow)
Conclusions
A wide variety of tumours and non-tumorous conditions involve the anorectal region apart from the common adenocarcinoma, inflammatory bowel disease, rectal varices and post-radiation proctitis. A summary of characteristic CT and MRI features are given in Table 1. Cross-sectional imaging, especially MRI, can play an important role in the detection and differentiation of these conditions, although biopsy is often needed for more specific histopathological diagnosis. And for the radiologists, knowledge of the existence of these spectrums of uncommon conditions and their differing imaging appearances is essential for accurate preoperative diagnosis, to differentiate from common rectal adenocarcinoma and thereby to help in appropriate management.
References
Rouse HC, Godoy MC, Lee WK, Phang PT, Brown CJ, Brown JA (2008) Imaging findings of unusual anorectal and perirectal pathology: a multi-modality approach. Clin Radiol 63(12):1350–1360
Gowdra Halappa V, Corona Villalobos CP, Bonekamp S et al (2012) Rectal imaging: part 1, High-resolution MRI of carcinoma of the rectum at 3 T. AJR Am J Roentgenol 199(1):W35–W42
Raghunathan G, Mortele KJ (2009) Magnetic resonance imaging of anorectal neoplasms. Clin Gastroenterol Hepatol 7(4):379–388
Kim SH, Lee JM, Lee MW, Kim GH, Han JK, Choi BI (2008) Sonography transmission gel as endorectal contrast agent for tumor visualization in rectal cancer. AJR Am J Roentgenol 191(1):186–189
Qayyum A (2009) Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics 29(6):1797–1810
Whittaker CS, Coady A, Culver L, Rustin G, Padwick M, Padhani AR (2009) Diffusion-weighted MR imaging of female pelvic tumors: a pictorial review. Radiographics 29(3):759–774
Bonekamp S, Corona-Villalobos CP, Kamel IR (2012) Oncologic applications of diffusion-weighted MRI in the body. J Magn Reson Imaging 35(2):257–279
Bozgeyik Z, Onur MR, Poyraz AK (2013) The role of diffusion weighted magnetic resonance imaging in oncologic settings. Quant Imaging Med Surg 3(5):269–278
Nougaret S, Tirumani SH, Addley H, Pandey H, Sala E, Reinhold C (2013) Pearls and pitfalls in MRI of gynecologic malignancy with diffusion-weighted technique. AJR Am J Roentgenol 200(2):261–276
Ichikawa T, Erturk SM, Motosugi U et al (2006) High-B-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol 187(1):181–184
Jung SH, Heo SH, Kim JW et al (2012) Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging 35(1):110–116
Sun YS, Zhang XP, Tang L et al (2010) Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 254(1):170–178
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11(2):102–125
Lin C, Luciani A, Itti E et al (2012) Whole-body diffusion magnetic resonance imaging in the assessment of lymphoma. Cancer Imaging 12:403–408
Wong CS, Gong N, Chu YC et al (2012) Correlation of measurements from diffusion weighted MR imaging and FDG PET/CT in GIST patients: ADC versus SUV. Eur J Radiol 81(9):2122–2126
Halefoglu AM, Sen EY (2012) Precoccygeal epidermal inclusion cyst: ultrasound and MR imaging features. JBR-BTR 95(5):294–296
Yang DM, Yoon MH, Kim HS et al (2001) Presacral epidermoid cyst: imaging findings with histopathologic correlation. Abdom Imaging 26(1):79–82
Dahan H, Arrive L, Wendum D, Docou le Pointe H, Djouhri H, Tubiana JM (2001) Retrorectal developmental cysts in adults: clinical and radiologic-histopathologic review, differential diagnosis, and treatment. Radiographics 21(3):575–584
Aflalo-Hazan V, Rousset P, Mourra N, Lewin M, Azizi L, Hoeffel C (2008) Tailgut cysts: MRI findings. Eur Radiol 18(11):2586–2593
Nallamothu G, Adler DG (2011) Large colonic lipomas. Gastroenterol Hepatol (N Y) 7(7):490–492
Pickhardt PJ, Kim DH, Menias CO, Gopal DV, Arluk GM, Heise CP (2007) Evaluation of submucosal lesions of the large intestine: part 1. Neoplasms. Radiographics 27(6):1681–1692
Kim H, Kim JH, Lim JS et al (2011) MRI findings of rectal submucosal tumors. Korean J Radiol 12(4):487–498
Chung JJ, Kim MJ, Lee JT, Yoo HS (2000) Large villous adenoma in rectum mimicking cerebral hemispheres. AJR Am J Roentgenol 175(5):1465–1466
Maciejewski A, Lange D, Wloch J (2000) Case report of schwannoma of the rectum–clinical and pathological contribution. Med Sci Monit 6(4):779–782
Tanaka N, Onda M, Seya T, Furukawa K, Kumazaki T (1999) Diffuse cavernous haemangioma of the rectum. Eur J Surg 165(3):280–283
Djouhri H, Arrive L, Bouras T, Martin B, Monnier-Cholley L, Tubiana JM (1998) MR imaging of diffuse cavernous hemangioma of the rectosigmoid colon. AJR Am J Roentgenol 171(2):413–417
Hussain SM, Outwater EK, Siegelman ES (1999) Mucinous versus nonmucinous rectal carcinomas: differentiation with MR imaging. Radiology 213(1):79–85
Chen JS, Hsieh PS, Hung SY et al (2004) Clinical significance of signet ring cell rectal carcinoma. Int J Colorectal Dis 19(2):102–107
Sasaki S, Masaki T, Umetani N, Futakawa N, Ando H, Muto T (1998) Characteristics in primary signet-ring cell carcinoma of the colorectum, from clinicopathological observations. Jpn J Clin Oncol 28(3):202–206
Rudralingam V, Dobson MJ, Pitt M, Stewart DJ, Hearn A, Susnerwala S (2003) MR imaging of linitis plastica of the rectum. AJR Am J Roentgenol 181(2):428–430
Lee HJ, Han JK, Kim TK et al (2002) Primary colorectal lymphoma: spectrum of imaging findings with pathologic correlation. Eur Radiol 12(9):2242–2249
Haroon S, Memon A, Pervez S (2013) Multiple lymphomatous polyposis form of blastoid variant of mantle cell lymphoma in colon: a case report and review of literature. J Gastrointest Cancer. doi:10.1007/s12029-013-9541-3
Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M (2003) Anorectal gastrointestinal stromal tumors: CT and MR imaging features with clinical and pathologic correlation. AJR Am J Roentgenol 180(6):1607–1612
Sandrasegaran K, Rajesh A, Rydberg J, Rushing DA, Akisik FM, Henley JD (2005) Gastrointestinal stromal tumors: clinical, radiologic, and pathologic features. AJR Am J Roentgenol 184(3):803–811
Hama Y, Okizuka H, Odajima K, Hayakawa M, Kusano S (2001) Gastrointestinal stromal tumor of the rectum. Eur Radiol 11(2):216–219
36 Kloppel G, Perren A, Heitz PU (2004) The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci:13–27.
Modlin IM, Lye KD, Kidd M (2003) A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97(4):934–959
Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD (2005) Current status of gastrointestinal carcinoids. Gastroenterology 128(6):1717–1751
Kantawala KP, Sonavane SK, Menias CO, Pai RK (2011) Atypical tumors of the rectum with pathologic correlation. Curr Probl Diagn Radiol 40(5):198–207
Rockall AG, Reznek RH (2007) Imaging of neuroendocrine tumours (CT/MR/US). Best Pract Res Clin Endocrinol Metab 21(1):43–68
Restrepo CS, Martinez S, Lemos JA et al (2006) Imaging manifestations of Kaposi sarcoma. Radiographics 26(4):1169–1185
Hoeffel CC, Azizi L, Mourra N, Lewin M, Arrive L, Tubiana JM (2006) MRI of rectal disorders. AJR Am J Roentgenol 187(3):W275–W284
Laoutliev B, Harling H, Neergaard K (2005) Simonsen L Rectal metastasis from infiltrating lobular breast carcinoma: imaging with 18 F-FDG PET. Eur Radiol 15(1):186–8
Kinkel K, Frei KA, Balleyguier C, Chapron C (2006) Diagnosis of endometriosis with imaging: a review. Eur Radiol 16(2):285–298
Puglielli E, Di Cesare E (2004) Masciocchi C Rectal endometriosis: MRI study with rectal coil. Eur Radiol 14(12):2362–3
Bazot M, Darai E, Hourani R et al (2004) Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology 232(2):379–389
Glastonbury CM (2002) The shading sign. Radiology 224(1):199–201
Heffernan C, Pachter HL, Megibow AJ, Macari M (2005) Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol 184(4):1189–1193
Wu CH, Wang LJ, Wong YC et al (2011) Necrotic stercoral colitis: importance of computed tomography findings. World J Gastroenterol 17(3):379–384
Maurer CA, Renzulli P, Mazzucchelli L, Egger B, Seiler CA, Buchler MW (2000) Use of accurate diagnostic criteria may increase incidence of stercoral perforation of the colon. Dis Colon Rectum 43(7):991–998
Matsuoka Y, Masumoto T, Suzuki K et al (1999) Pseudomyxoma retroperitonei. Eur Radiol 9(3):457–459
Lee NK, Kim S, Kim HS et al (2011) Spectrum of mucin-producing neoplastic conditions of the abdomen and pelvis: cross-sectional imaging evaluation. World J Gastroenterol 17(43):4757–4771
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Virmani, V., Ramanathan, S., Virmani, V.S. et al. What is hiding in the hindgut sac? Looking beyond rectal carcinoma. Insights Imaging 5, 457–471 (2014). https://doi.org/10.1007/s13244-014-0347-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13244-014-0347-z