Abstract
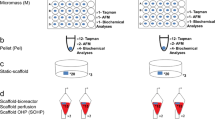
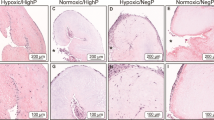
Engineered valvular tissues are cultured dynamically, and involve specimen movement. We previously demonstrated that oscillatory shear stresses (OSS) under combined steady flow and specimen cyclic flexure (flex-flow) promote tissue formation. However, localized efficiency of specimen mass transport is also important in the context of cell viability within the growing tissues. Here, we investigated the delivery of two essential species for cell survival, glucose and oxygen, to 3-dimensional (3D) engineered valvular tissues. We applied a convective-diffusive model to characterize glucose and oxygen mass transport with and without valve-like specimen flexural movement. We found the mass transport effects for glucose and oxygen to be negligible for scaffold porosities typically present during in vitro experiments and non-essential unless the porosity was unusually low (<40%). For more typical scaffold porosities (75%) however, we found negligible variation in the specimen mass fraction of glucose and oxygen in both non-moving and moving constructs (p > 0.05). Based on this result, we conducted an experiment using bone marrow stem cell (BMSC)-seeded scaffolds under Pulsatile flow-alone states to permit OSS without any specimen movement. BMSC-seeded specimen collagen from the pulsatile flow and flex-flow environments were subsequently found to be comparable (p > 0.05) and exhibited some gene expression similarities. We conclude that a critical magnitude of fluid-induced, OSS created by either pulsatile flow or flex-flow conditions, particularly when the oscillations are physiologically-relevant, is the direct, principal stimulus that promotes engineered valvular tissues and its phenotype, whereas mass transport benefits derived from specimen movement are minimal.




Similar content being viewed by others
References
Abraham, J., et al. A mass transfer model of temporal drug deposition in artery walls. Int. J. Heat Mass Transf. 58(1):632–638, 2013.
Ai, L., and K. Vafai. A coupling model for macromolecule transport in a stenosed arterial wall. Int. J. Heat Mass Transf. 49(9):1568–1591, 2006.
Boubriak, O., J. Urban, and Z. Cui. Monitoring of lactate and glucose levels in engineered cartilage construct by microdialysis. J. Membr. Sci. 273(1):77–83, 2006.
Brown, D. A., et al. Analysis of oxygen transport in a diffusion-limited model of engineered heart tissue. Biotechnol. Bioeng. 97(4):962–975, 2007.
Chen, G.-Q., and Q. Wu. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26(33):6565–6578, 2005.
Chen, J., et al. Numerical investigation of mass transport through patient-specific deformed aortae. J. Biomech. 47(2):544–552, 2014.
Chung, S., and K. Vafai. Low-density lipoprotein transport within a multi-layered arterial wall—effect of the atherosclerotic plaque/stenosis. J. Biomech. 46(3):574–585, 2013.
Chung, S., and K. Vafai. Mechanobiology of low-density lipoprotein transport within an arterial wall—Impact of hyperthermia and coupling effects. J. Biomech. 47(1):137–147, 2014.
Converti, A., et al. Evaluation of glucose diffusion coefficient through cell layers for the kinetic study of an immobilized cell bioreactor. Chem. Eng. Sci. 51(7):1023–1026, 1996.
Das, D. B. Multiscale simulation of nutrient transport in hollow fibre membrane bioreactor for growing bone tissue: sub-cellular scale and beyond. Chem. Eng. Sci. 62(13):3627–3639, 2007.
Day, P., et al. What factors determine placental glucose transfer kinetics? Placenta 34(10):953–958, 2013.
De Monte, F., G. Pontrelli, and S. Becker. Drug release in biological tissues. Transport in biological media. Amsterdam: Elsevier Science BV, 2013.
Dohmen, P. M., et al. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann. Thorac. Surg. 92(4):1308–1314, 2011.
Engelmayr, G. C., et al. A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials. Biomaterials 24(14):2523–2532, 2003.
Engelmayr, G. C., et al. Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: implications for engineered heart valve tissues. Biomaterials 27(36):6083–6095, 2006.
Engelmayr, Jr, G. C., et al. A novel flex-stretch-flow bioreactor for the study of engineered heart valve tissue mechanobiology. Ann. Biomed. Eng. 36(5):700–712, 2008.
Fong, P., et al. The use of polymer based scaffolds in tissue-engineered heart valves. Prog Pediatr Cardiol 21(2):193–199, 2006.
Frank, B. S., et al. Determining cell seeding dosages for tissue engineering human pulmonary valves. J. Surg. Res. 174(1):39–47, 2012.
Gao, Z., and C. W. Xu. Glucose metabolism induces mono-ubiquitination of histone H2B in mammalian cells. Biochem. Biophys. Res. Commun. 404(1):428–433, 2011.
Gill, J., et al. Modeling oxygen transport in human placental terminal villi. J. Theor. Biol. 291:33–41, 2011.
Hilfiker, A., et al. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbeck’s Arch. Surg. 396(4):489–497, 2011.
Hoerstrup, S. P., et al. Fluorescence activated cell sorting: a reliable method in tissue engineering of a bioprosthetic heart valve. Ann. Thorac. Surg. 66(5):1653–1657, 1998.
Hoerstrup, S. P., et al. Tissue engineering of a bioprosthetic heart valve: stimulation of extracellular matrix assessed by hydroxyproline assay. ASAIO J. 45(5):397, 1999.
Hoerstrup, S. P., et al. Functional living trileaflet heart valves grown in vitro. Circulation 102(suppl 3):Iii-44–Iii-49, 2000.
Hoerstrup, S. P., et al. New pulsatile bioreactor for in vitro formation of tissue engineered heart valves. Tissue Eng. 6(1):75–79, 2000.
Hoffman, J. Incidence of congenital heart disease: I Postnatal incidence. Pediatr. Cardiol. 16(3):103–113, 1995.
Ishaug-Riley, S. L., et al. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials 19(15):1405–1412, 1998.
Johnson, A. S., et al. Oxygen consumption and diffusion in assemblages of respiring spheres: performance enhancement of a bioartificial pancreas. Chem. Eng. Sci. 64(22):4470–4487, 2009.
Kaazempur-Mofrad, M., et al. Mass transport and fluid flow in stenotic arteries: axisymmetric and asymmetric models. Int. J. Heat Mass Transf. 48(21):4510–4517, 2005.
Kadner, A., et al. A new source for cardiovascular tissue engineering: human bone marrow stromal cells. Eur. J. Cardiothorac. Surg. 21(6):1055–1060, 2002.
Karner, G., and K. Perktold. Effect of endothelial injury and increased blood pressure on albumin accumulation in the arterial wall: a numerical study. J. Biomech. 33(6):709–715, 2000.
Khakpour, M., and K. Vafai. Critical assessment of arterial transport models. Int. J. Heat Mass Transf. 51(3):807–822, 2008.
Koutny, T. Estimating reaction delay for glucose level prediction. Med. Hypotheses 77(6):1034–1037, 2011.
Kumar, R., F. Stepanek, and A. Mantalaris. An oxygen transport model for human bone marrow microcirculation. Food Bioprod. Process. 82(2):105–116, 2004.
Leor, J., Y. Amsalem, and S. Cohen. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol. Ther. 105(2):151–163, 2005.
Lichtenberg, A., et al. Cell seeded tissue engineered cardiac valves based on allograft and xenograft scaffolds. Prog. Pediatr. Cardiol. 21(2):211–217, 2006.
Liu, X., et al. Effect of non-Newtonian and pulsatile blood flow on mass transport in the human aorta. J. Biomech. 44(6):1123–1131, 2011.
Lotz, J., et al. Cardiovascular flow measurement with phase-contrast MR imaging: basic facts and implementation 1. Radiographics 22(3):651–671, 2002.
Martineau, L. C. Large enhancement of skeletal muscle cell glucose uptake and suppression of hepatocyte glucose-6-phosphatase activity by weak uncouplers of oxidative phosphorylation. Biochim. Biophys. Acta. 1820(2):133–150, 2012.
Martinez, C., et al. Periodontal ligament cells cultured under steady-flow environments demonstrate potential for use in heart valve tissue engineering. Tissue Eng. Part A 19(3–4):458–466, 2012.
Moore, J., and C. Ethier. Oxygen mass transfer calculations in large arteries. J. Biomech. Eng. 119(4):469–475, 1997.
Neuenschwander, S., and S. P. Hoerstrup. Heart valve tissue engineering. Transpl. Immunol. 12(3):359–365, 2004.
Patnaik, S. S., et al. Decellularized scaffolds: concepts, methodologies, and applications in cardiac tissue engineering and whole-organ regeneration. Tissue regeneration: where nanostructure meets biology. 77–124, 2013.
Perry, T. E., et al. Bone marrow as a cell source for tissue engineering heart valves. Ann. Thorac. Surg. 75(3):761–767, 2003.
Prosi, M., et al. Mathematical and numerical models for transfer of low-density lipoproteins through the arterial walls: a new methodology for the model set up with applications to the study of disturbed lumenal flow. J. Biomech. 38(4):903–917, 2005.
Ramaswamy, S., et al. The role of organ level conditioning on the promotion of engineered heart valve tissue development in vitro using mesenchymal stem cells. Biomaterials 31(6):1114–1125, 2010.
Ramaswamy, S., et al. A novel bioreactor for mechanobiological studies of engineered heart valve tissue formation under pulmonary arterial physiological flow conditions. J. Biomech. Eng. 136(12):121009, 2014.
Rath, S., et al. Differentiation and distribution of marrow stem cells in flex-flow environments demonstrate support of the valvular phenotype. PLoS ONE 10(11):e0141802, 2015.
Reddy, J., V. U. Unnikrishnan, and G. U. Unnikrishnan. Recent advances in the analysis of nanotube-reinforced polymeric biomaterials. J. Mech. Behav. Mater. 22(5–6):137–148, 2013.
Roger, V. L., et al. Heart disease and stroke statistics—2011 update a report from the American Heart Association. Circulation 123(4):e18–e209, 2011.
Ruel, J., and G. Lachance. A new bioreactor for the development of tissue-engineered heart valves. Ann. Biomed. Eng. 37(4):674–681, 2009.
Salinas, M., and S. Ramaswamy. Computational simulations predict a key role for oscillatory fluid shear stress in de novo valvular tissue formation. J. Biomech. 47(14):3517–3523, 2014.
Salinas, M., et al. Oscillatory shear stress created by fluid pulsatility versus flexed specimen configurations. Comput. Method Biomech. Biomed. Eng. 17(7):728–739, 2014.
Schmidt, D., et al. Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng. 12(11):3223–3232, 2006.
Schmidt, D., et al. Minimally-invasive implantation of living tissue engineered heart valves: a comprehensive approach from autologous vascular cells to stem cells. J. Am. Coll. Cardiol. 56(6):510–520, 2010.
Sengers, B., et al. Computational study of culture conditions and nutrient supply in cartilage tissue engineering. Biotechnol. Prog. 21(4):1252–1261, 2005.
Siepe, M., et al. Stem cells used for cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 34(2):242–247, 2008.
Simon, P., et al. Tissue Engineering of heart valves—Immunologic and inflammatory challenges of the allograft scaffold. Prog. Pediatr. Cardiol. 21(2):161–165, 2006.
Sodian, R., et al. Tissue engineering of a trileaflet heart valve-early in vitro experiences with a combined polymer. Tissue Eng. 5(5):489, 1999.
Sodian, R., et al. Early in vivo experience with tissue-engineered trileaflet heart valves. Circulation 102(19 Suppl 3):III22, 2000.
Sodian, R., et al. Tissue engineering of heart valves: in vitro experiences. Ann. Thorac. Surg. 70(1):140, 2000.
Sodian, R., et al. Fabrication of a trileaflet heart valve scaffold from a polyhydroxyalkanoate biopolyester for use in tissue engineering. Tissue Eng. 6(2):183, 2000.
Soukane, D. M., A. Shirazi-Adl, and J. Urban. Computation of coupled diffusion of oxygen, glucose and lactic acid in an intervertebral disc. J. Biomech. 40(12):2645–2654, 2007.
Stangeby, D. K., and C. R. Ethier. Coupled computational analysis of arterial ldl transport—effects of hypertension. Comput. Method Biomech. Biomed. Eng. 5(3):233, 2002.
Stella, J. A., J. Liao, and M. S. Sacks. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. J. Biomech. 40(14):3169, 2007.
Sutherland, F. W. H., et al. From stem cells to viable autologous semilunar heart valve. Circulation 111(21):2783, 2005.
Tarbell, J. M., M. J. Lever, and C. G. Caro. The effect of varying albumin concentration of the hydraulic conductivity of the rabbit common carotid artery. Microvasc. Res. 35(2):204, 1988.
Unnikrishnan, G. U., V. U. Unnikrishnan, and J. N. Reddy. Finite element model for nutrient distribution analysis of a hollow fiber membrane bioreactor. Int. J. Numer. Methods Biomed. Eng. 28(2):229, 2012.
Vermot, J., et al. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart (reversing blood flows during valvulogenesis). PLoS Biol. 7(11):e1000246, 2009.
Wang, L., et al. Computational simulation of oxygen diffusion in aortic valve leaflet for tissue engineering applications. J. Heart valve Dis. 17(6):700, 2008.
Wang, L., et al. Factors influencing the oxygen consumption rate of aortic valve interstitial cells: application to tissue engineering (report). Tissue Eng. Part C Method 15(3):355, 2009.
Wang, L., et al. Prediction of oxygen distribution in aortic valve leaflet considering diffusion and convection. J. Heart valve Dis. 20(4):442, 2011.
Weber, B., S. M. Zeisberger, and S. P. Hoerstrup. Prenatally harvested cells for cardiovascular tissue engineering: fabrication of autologous implants prior to birth. Placenta 32:S316, 2011.
Weber, B., et al. Engineering of living autologous human umbilical cord cell-based septal occluder membranes using composite PGA-P4HB matrices. Biomaterials 32(36):9630, 2011.
Yang, N., and K. Vafai. Modeling of low-density lipoprotein (LDL) transport in the artery—effects of hypertension. Int. J. Heat Mass Transf. 49(5):850, 2006.
Yu, P., et al. Fluid dynamics and oxygen transport in a micro-bioreactor with a tissue engineering scaffold. Int. J. Heat Mass Transf. 52(1):316, 2009.
Acknowledgments
The authors acknowledge funds received from the department of Biomedical Engineering at Florida International University to carry out this research work. Funding for M.S. by NIH/NIGMS R25 GM061347 is gratefully acknowledged.
Conflict of interest
The authors have no conflict of interests.
Statement of Human and Animal Studies
No Human and Animal Studies were involved in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Salinas, M., Rath, S., Villegas, A. et al. Relative Effects of Fluid Oscillations and Nutrient Transport in the In Vitro Growth of Valvular Tissues. Cardiovasc Eng Tech 7, 170–181 (2016). https://doi.org/10.1007/s13239-016-0258-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-016-0258-x