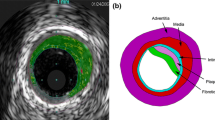
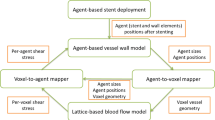
Abstract
Structural and fluid stresses acting on the artery wall are proposed as mechanical mediators of in-stent restenosis (ISR). This study reports an investigation of the correlation between stresses obtained from computational simulations with the magnitude of ISR at the level of individual stent struts observed in an in vivo model of restenosis. Structural and fluid dynamic analyses were undertaken in a model based on volumetric micro-CT data from an in vivo stent deployment in a porcine right coronary artery. Structural and fluid mechanics were compared with histological data from the same stented vessel sample. Interpretation of the combined data at the level of individual stent struts was possible by identifying the location of each 2-D histological section within the 3-D micro-CT volume. Linear correlation between structural and fluid stimuli and neointimal thickness at the level of individual struts is less clear when individual stimuli are considered [compressive force (CF), R 2 = 0.19, wall shear stress (WSS), R 2 = 0.25, oscillatory shear index (OSI), R 2 = 0.28]. Closer correlation is observed if combined structural and fluid stimuli are assumed to stimulate ISR (CF/WSS, R 2 = 0.64). The use of micro-CT to characterise stent geometry after deployment enhances the clinical relevance of computational simulations, allowing direct comparison with histology. The results support the combined role of both structural and fluid mechanics to determine the magnitude of ISR at the level of individual struts. This finding is consistent with other studies which consider these stimuli averaged over a transverse section of the vessel.






Similar content being viewed by others
References
Bennett, M. R. In-stent stenosis: pathology and implications for the development of drug eluting stents. Heart 89(2):218–224, 2003.
Boyle, C. J., A. B. Lennon, and P. J. Prendergast. Application of a mechanobiological simulation technique to stents used clinically. J. Biomech. 46(5):918–924, 2013.
Boyle, C. J., et al. Computational simulation methodologies for mechanobiological modelling: a cell-centred approach to neointima development in stents. Philos. Trans. R. Soc. A 2010(368):2919–2935, 1921.
Chaabane, C., et al. Biological responses in stented arteries. Cardiovasc. Res. 99(2):353–363, 2013.
Chen, H. Y., et al. Mis-sizing of stent promotes intimal hyperplasia: impact of endothelial shear and intramural stress. Am. J. Physiol. Heart Circ. Physiol. 301(6):H2254–H2263, 2011.
Chiastra, C., et al. Computational fluid dynamics of stented coronary bifurcations studied with a hybrid discretization method. Eur. J. Mech. B 35:76–84, 2012.
Chiu, J–. J., and S. Chien. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91(1):327–387, 2011.
Chiu, J–. J., et al. A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J. Biomech. 37(4):531–539, 2004.
Colombo, A., et al. Cyclic strain amplitude dictates the growth response of vascular smooth muscle cells in vitro: role in in-stent restenosis and inhibition with a sirolimus drug-eluting stent. Biomech. Model. Mechanobiol. 212(4):671–683, 2013.
Conway, C., et al. A computational test-bed to assess coronary stent implantation mechanics using a population-specific approach. Cardiovasc. Eng. Technol. 3(4):374–387, 2012.
Duraiswamy, N., et al. Spatial distribution of platelet deposition in stented arterial models under physiologic flow. Ann. Biomed. Eng. 33(12):1767–1777, 2005.
Edelman, E. R., and C. Rogers. Pathobiologic responses to stenting. Am. J. Cardiol. 81(7, Supplement 1):4E–6E, 1998.
Gijsen, F. J., et al. Simulation of stent deployment in a realistic human coronary artery. BioMed. Eng. OnLine 7(23):1–11, 2008.
Gogas, B. D., et al. The edge vascular response following implantation of the absorb everolimus-eluting bioresorbable vascular scaffold and the XIENCE V metallic everolimus-eluting stent. First serial follow-up assessment at six months and two years: insights from the first-in-man ABSORB Cohort B and SPIRIT II trials. EuroIntervention 9(6):709–720, 2013.
Gojova, A., and A. I. Barakat. Vascular endothelial wound closure under shear stress: role of membrane fluidity and flow-sensitive ion channels. J. Appl. Physiol. 98(6):2355–2362, 2005.
Gunn, J., et al. Coronary artery stretch versus deep injury in the development of in-stent neointima. Heart 88(4):401–405, 2002.
Huo, Y., et al. Effects of vessel compliance on flow pattern in porcine epicardial right coronary arterial tree. J. Biomech. 42(5):594–602, 2009.
Kleinstreuer, C., et al. Hemodynamic parameters and early intimal thickening in branching blood vessels. Crit. Rev. Biomed. Eng. 29(1):1–64, 2001.
Kleinstreuer, C., et al. Hemodynamic parameters and early intimal thickening in branching blood vessels. Crit. Rev. Biomed. Eng. 29(1):1–64, 2001.
Kolandavel, M., et al. The effects of time varying curvature on species transport in coronary arteries. Ann. Biomed. Eng. 34(12):1820–1832, 2006.
Koskinas, K. C., et al. Role of endothelial shear stress in stent restenosis and thrombosis: pathophysiologic mechanisms and implications for clinical translation. J. Am. Coll. Cardiol. 59(15):1337–1349, 2012.
Kroll, M., et al. Platelets and shear stess. Blood 88(5):1525–1541, 1996.
LaDisa, J. F., et al. Stent implantation alters coronary artery hemodynamics and wall shear stress during maximal vasodilation. J. Appl. Physiol. 93(6):1939–1946, 2002.
LaDisa, Jr., J. F., et al. Alterations in wall shear stress predict sites of neointimal hyperplasia after stent implantation in rabbit iliac arteries. Am. J. Physiol. Heart Circ. Physiol. 288(5):H2465–H2475, 2005.
LaDisa, J. F., et al. Alterations in regional vascular geometry produced by theoretical stent implantation influence distributions of wall shear stress: analysis of a curved coronary artery using 3D computational fluid dynamics modeling. BioMed. Eng. OnLine 5(40):11, 2006.
Linder-Ganz, E., et al. Pressure–time cell death threshold for albino rat skeletal muscles as related to pressure sore biomechanics. J. Biomech. 39(14):2725–2732, 2006.
Malek, A. M., S. L. Alper, and S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282(21):2035–2042, 1999.
Malek, A., and S. Izumo. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am. J. Physiol. Cell Physiol. 263(2):C389–C396, 1992.
Messenger, J., et al. 3D coronary reconstruction from routine single-plane coronary angiograms: clinical validation and quantitative analysis of the right coronary artery in 100 patients. Int. J. Card. Imaging 16(6):413–427, 2000.
Moazzam, F., et al. The leukocyte response to fluid stress. Proc. Natl. Acad. Sci. 94(10):5338–5343, 1997.
Moore, Jr., J., and J. L. Berry. Fluid and solid mechanical implications of vascular stenting. Ann. Biomed. Eng. 30(4):498–508, 2002.
Morlacchi, S., et al. Sequential structural and fluid dynamic numerical simulations of a stented bifurcated coronary artery. J. Biomech. Eng. 133(12):1–11, 2011.
Morlacchi, S., et al. Hemodynamics and in-stent restenosis: micro-CT images, histology, and computer simulations. Ann. Biomed. Eng. 39(10):2615–2626, 2011.
Murphy, J., and F. Boyle. Predicting neointimal hyperplasia in stented arteries using time-dependant computational fluid dynamics: a review. Comput. Biol. Med. 40(4):408–418, 2010.
Nichols, W. W., and M. F. O’Rourke. McDonald’s blood flow in arteries (3rd ed.). Philadelphia: Lea & Febiger, 1990.
Sanmartín, M., et al. Influence of shear stress on in-stent restenosis: in vivo study using 3D reconstruction and computational fluid dynamics. Revista Española de Cardiología 59(1):20–27, 2006.
Selvarasu, N. K. C., and D. K. Tafti. Investigation of the effects of dynamic change in curvature and torsion on pulsatile flow in a helical tube. J. Biomech. Eng. 134(7):071005–071017, 2012.
Seo, T., L. G. Schachter, and A. I. Barakat. Computational study of fluid mechanical disturbance induced by endovascular stents. Ann. Biomed. Eng. 33(4):444–456, 2005.
Sharpe, J., et al. Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296(5567):541–545, 2002.
Stefanini, G. G., and D. R. Holmes, Jr. Drug-eluting coronary-artery stents. N. Engl. J. Med. 368(3):254–265, 2013.
Stettler, C., et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370(9591):937–948, 2007.
Tahir, H., et al. Multi-scale simulations of the dynamics of in-stent restenosis: impact of stent deployment and design. Interface Focus 1(3):365–373, 2011.
Thury, A., et al. Focal in-stent restenosis near step-up. Circulation 105(23):e185–e187, 2002.
Timmins, L. H., et al. Increased artery wall stress post-stenting leads to greater intimal thickening. Lab. Investig. 91(6):955–967, 2011.
Wang, J., and B. Thampatty. An introductory review of cell mechanobiology. Biomech. Model. Mechanobiol. 5(1):1–16, 2006.
Acknowledgments
We gratefully acknowledge financial support from the EU in the form of a Marie Curie fellowship (Project MeDDiCA Marie Curie Initial Training Network, www.meddica.eu, EU-FP7/2007-2013 under Grant agreement PITN-GA-2008-238113).
Conflict of interest
Brandis Keller, Claudia Amatruda, D Rodney Hose, Julian Gunn, Patricia Lawford, Gabriele Dubini, Francesco Migliavacca and Andrew Narracott declare that they have no conflict of interest.
Ethical standards
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees. The work reported in this manuscript does not involve Human Subjects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Brandis K. Keller and Claudia M Amatruda shared first authorship.
Rights and permissions
About this article
Cite this article
Keller, B.K., Amatruda, C.M., Hose, D.R. et al. Contribution of Mechanical and Fluid Stresses to the Magnitude of In-stent Restenosis at the Level of Individual Stent Struts. Cardiovasc Eng Tech 5, 164–175 (2014). https://doi.org/10.1007/s13239-014-0181-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-014-0181-y