Abstract
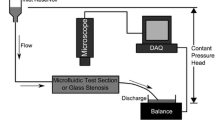
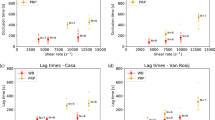
Most previous studies have investigated in vitro thrombus formation under steady flow conditions at physiological shear rates, though occlusive thrombosis leading to myocardial infarction and stroke forms under elevated shear rates and pulsatile flow. Two reports of pulsatile flow on thrombosis have yielded conflicting results. In the present study, we quantify the effect of very high shear, reversing pulsatile flow relevant to coronary thrombosis on platelet deposition leading to occlusive thrombus formation. Whole porcine blood was perfused in a collagen-coated, tubular, stenotic test section under pulsatile or steady flow. Pulsatile flow was generated with a frequency of 60 beats per minute and large magnitude excursions similar to a coronary artery waveform. Alternatively, steady flow conditions from a pressure driven system created shear rates matched to the maximum (16000 s−1), mean (3800 s−1), and an intermediate (6500 s−1) shear rates corresponding to the pulsatile system. Thrombus growth in thickness was recorded using a high-resolution CCD attached to a microscope. Steady flow recreated pulsatile flow thrombus formation in most cases. Lag time, t lag, thrombus growth rate, dV/dt, and time to occlusion, t occ, did not show statistically significant differences between pulsatile flow and steady flow with matched mean shear rate. Pulsatile flow conditions yielded t occ = 5.5 ± 2.8 min, which was not significantly different compared to a steady mean shear rate condition with t occ = 6.2 ± 1.7 min. Similarly, occlusion times for steady intermediate and steady maximum shear rate conditions were not significantly difference from pulsatile flow conditions yielding t occ of 4.6 ± 2.9 and 4.6 ± 1.8 min, respectively. In contrast, lag time for steady flow at maximum shear rate of 16000 s−1 was decreased to 26.1 s compared to pulsatile flow (t lag = 42.7 s, p = 0.03), steady mean flow (t lag = 60.9 s, p = 0.02), and steady intermediate flow (t lag = 39.9 s, p = 0.01). Occlusive thrombus formation under high shear, pulsatile conditions may be modeled in vitro using steady flow with matched mean shear rate with respect to occlusion time, lag time, and growth rate. Our results indicate that the magnitude of shear rate more strongly affects thrombus growth characteristics than flow pulsatility for an arterial frequency.






Similar content being viewed by others
References
Aarts, P. A., et al. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arterioscler. Thromb. Vasc. Biol. 8:819–824, 1988.
Badimon, L., and J. J. Badimon. Mechanisms of arterial thrombosis in nonparallel streamlines: platelet thrombi grow on the apex of stenotic severely injured vessel wall, experimental study in a pig model. J. Clin. Investig. 84:1134–1144, 1989.
Badimon, J. J., et al. Influence of arterial damage and wall shear rate on platelet deposition: ex vivo study in a swine model. Arterioscler. Thromb. Vasc. Biol. 6:312–320, 1986.
Bark, Jr, D. L., and D. N. Ku. Wall shear over high degree stenoses pertinent to atherothrombosis. J. Biomech. 43:2970–2977, 2010.
Bark, D., A. Para, and D. Ku. Correlation of thrombosis growth rate to pathological wall shear rate during platelet accumulation. Biotechnol. Bioeng. 109:2642–2650, 2012.
Barstad, R. M., et al. A perfusion chamber developed to investigate thombus formation and shear profiles in flowing native human blood at the apex of a well-defined stenosis. Arterioscler. Thromb. Vasc. Biol. 14:1984–1991, 1994.
Colace, T., J. Jobson, and S. L. Diamond. Relipidated tissue factor linked to collagen surfaces potentiates platelet adhesion and fibrin formation in a microfluidic model of vessel injury. Bioconjug. Chem. 22:2104–2109, 2011.
Davies, M. J. Pathology of arterial thrombosis. Br. Med. Bull. 50:789–802, 1994.
Davies, M. J., W. Fulton, and W. Robertson. The relation of coronary thrombosis to ischaemic myocardial necrosis. J. Pathol. 127:99–110, 1979.
Davies, M. J., and A. Thomas. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N. Engl. J. Med. 310:1137–1140, 1984.
Davies, M. J., and A. Thomas. Plaque fissuring: the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br. Heart J. 53:363–373, 1985.
Eckstein, E., A. Tilles, and F. Milero. Conditions for the occurrence of large near-wall excesses of small particles during blood flow. Microvasc. Res. 36:31–39, 1988.
Edelstein, A., et al. Computer control of microscopes using & μManager. New York: Wiley, 2010.
Epstein, F. H., et al. Mechanisms of disease: platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. N. Engl. J. Med. 332:1553–1559, 1995.
Gutierrez, E., et al. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip 8:1486–1495, 2008.
Hellums, J. 1993 Whitaker Lecture: biorheology in thrombosis research. Ann. Biomed. Eng. 22:445–455, 1994.
Holme, P. A., et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler. Thromb. Vasc. Biol. 17:646–653, 1997.
Hosokawa, K., et al. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J. Thromb. Haemost. JTH 9:2029–2037, 2011.
Houdijk, W. P., et al. Subendothelial proteins and platelet adhesion: von Willebrand factor and fibronectin, not thrombospondin, are involved in platelet adhesion to extracellular matrix of human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 6:24–33, 1986.
Jackson, S. P. The growing complexity of platelet aggregation. Blood 109:5087–5095, 2007.
Jordan, A., et al. The effects of margination and red cell augmented platelet diffusivity on platelet adhesion in complex flow. Biorheology 41:641, 2004.
Kenner, T. The measurement of blood density and its meaning. Basic Res. Cardiol. 84:111–124, 1989.
Ku, D. N. Blood flow in arteries. Ann. Rev. Fluid Mech. 29:399–434, 1997.
Ku, D. N., and C. J. Flannery. Development of a flow-through system to create occluding thrombus. Biorheology 44:273–284, 2007.
Leighton, D., and A. Acrivos. The shear-induced migration of particles in concentrated suspension. J. Fluid Mech. 181:415–439, 1987.
Li, M., D. Ku, and C. Forest. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip 12:1355–1362, 2012.
Mailhac, A., et al. Effect of an eccentric severe stenosis on fibrin(ogen) deposition on severely damaged vessel wall in arterial thrombosis: relative contribution of fibrin(ogen) and platelets. Circulation 90:988–996, 1994.
Maloney, S. F., L. Brass, and S. L. Diamond. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integr. Biol. 2:153–220, 2010.
Marr, D., and E. Hildreth. Theory of edge detection. Proc. R. Soc. B 207:187–217, 1980.
Merrill, E. W. Rheology of blood. Physiol. Rev. 49:863–888, 1969.
Neeves, K., et al. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: pAR4 signaling enhances shear-resistance of platelet aggregates. J. Thromb. Haemost. 6:2193–2201, 2008.
Neeves, K. B., et al. Sources of variability in platelet accumulation on type 1 fibrillar collagen in microfluidic flow assays. PLoS One 8:e54680, 2013.
Para, A. N., and D. N. Ku. A low-volume, single pass in vitro system of high shear thrombosis in a stenosis. Thromb. Res. 131:418–424, 2013.
Para, A., et al. Rapid platelet accumulation leading to thrombotic occlusion. Ann. Biomed. Eng. 39:1961–1971, 2011.
Ramstack, J., L. Zuckerman, and L. Mockros. Shear-induced activation of platelets. J. Biomech. 12:113–125, 1979.
Replogle, R. L., H. J. Meiselman, and E. W. Merrill. Clinical implications of blood rheology studies. Circulation 36:148–160, 1967.
Ruggeri, Z. M. Von Willebrand factor: looking back and looking forward. Thromb. Haemost. 98(1):55–62, 2007.
Ruggeri, Z. M., et al. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 108:1903–1910, 2006.
Sakariassen, K. S., P. Aarts, and P. G. De Groot. A perfusion chamber developed to investigate platelet interaction in flowing blood with human vessel wall cells, their extracellular matrix, and purified components. J. Lab. Clin. Med. 102:522–535, 1983.
Savage, B., E. Saldivar, and Z. M. Ruggeri. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 84:289–297, 1996.
Sixma, J., et al. A new perfusion chamber to detect platelet adhesion using a small volume of blood. Thromb. Res. 92:S43–S46, 1998.
Taylor, G. Dispersion of soluble matter in solvent flowing slowly through a tube. Proc. R. Soc. A 219:186–203, 1953.
Van Breugel, H. H., J. J. Sixma, and R. M. Heethaar. Effects of flow pulsatility on platelet adhesion to subendothelium. Arterioscler. Thromb. Vasc. Biol. 8:332–335, 1988.
Velik-Salchner, C., et al. Normal values for thrombelastography (ROTEM) and selected coagulation parameters in porcine blood. Thromb. Res. 117:597–602, 2006.
Wellings, P. J., and D. N. Ku. Mechanisms of platelet capture under very high shear. Cardiovasc. Eng. Technol. 3:161–170, 2012.
Xu, C., and D. M. Wootton. Platelet near-wall excess in porcine whole blood in artery-sized tubes under steady and pulsatile flow conditions. Biorheology 41:113–125, 2004.
Zhao, X. M., et al. The influence of the pulsatility of the blood flow on the extent of platelet adhesion. Thromb. Res. 121:821–825, 2008.
Zydney, A., and C. Colton. Augmented solute transport in the shear flow of a concentrated suspension. Physiochem. Hydrodyn. 10:77–96, 1988.
Acknowledgments
LDCC was supported by DoD, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a.
Conflict of interest
LDCC and DNK declare that they have no conflicts of interest.
Statement of Human Studies
No animal studies were carried out by the authors for this article. Porcine blood samples were obtained as by-products of commercial animal slaughter.
Statement of Animal Rights
LDCC and DNK declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Appendix
Appendix
Reynolds Number
The Reynolds number (Re) is the non-dimensional ratio of inertial forces to viscous forces. For this study, Re was defined as
where ρ is the fluid density, L is the diameter of the test section, U mean is the mean velocity at the throat, and μ is the fluid viscosity. The density and viscosity of blood were taken as 1050 kg/m3 and 3.24 × 10−3 Pa s, respectively.22,30 Outside the stenosis, the diameter was 1.5 mm and the average velocity ranged from 0.0075 to 0.034 m/s across the three steady flow conditions, yielding a Reynolds number between 3.6 and 16.5. Inside the stenosis throat, an average diameter of 325 μm was used, and the average velocity ranged from 0.16 to 0.687 m/s, yielding a Reynolds number between 17 and 72.
Schmidt Number
The Schmidt number (Sc) is the non-dimensional ratio of momentum diffusivity to mass diffusivity, and is defined as
where D is the diffusivity of platelets. Platelet diffusivity may be considered either as the diffusivity of platelets in plasma, or as the effective diffusivity of platelets in blood under flow. Platelet diffusivity in plasma is 1 × 10−13 m2/s.21 However, since blood is a dense suspension of cells, effective platelet diffusivity increases with shear rate. Effective diffusivity (D eff) cab be estimated by21,48
where D sf is platelet diffusion in plasma (1 × 10−13 m2/s), a is the radius of the red blood cells (4.2 μm), γ is the shear rate, and Φ is the hematocrit (0.4). Using this equation, the maximum platelet diffusivity using the wall shear rates is 8.3 × 10−8 m2/s. Substituting the values for diffusivity into Eq. (5) gives a maximum Sc at the throat of the stenosis for the no-shear condition of 3.7 × 106, and effective Sc between 154 for the mean shear condition and 37 for the maximum shear condition. This range of Sc reflects that platelet diffusion is slower than fluid momentum transfer.
Peclet Number
The Peclet number (Pe) is the non-dimensional ratio of advective transport rate to diffusive transport rate. Pe is defined as
Multiplying the above values for Re and Sc for the various experimental conditions yields a Pe at the throat of the stenosis of between 6.2 × 107 and 2.6 × 108 for the minimum diffusivity, and between 2618 and 2664 using values for effective diffusivity. Such high Peclet numbers suggest that the platelet mass transport is dominated by the fluid flow and not simple diffusion.
Rights and permissions
About this article
Cite this article
Casa, L.D.C., Ku, D.N. High Shear Thrombus Formation under Pulsatile and Steady Flow. Cardiovasc Eng Tech 5, 154–163 (2014). https://doi.org/10.1007/s13239-014-0180-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-014-0180-z