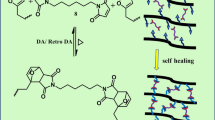
Abstract
At various temperatures, real time FTIR analysis was introduced to monitor the kinetics of the uncatalyzed Huisgen 1,3-dipolar azide-alkyne cycloaddition (AAC) without media, resulting in in situ robust polymeric networks crosslinked with triazoles at chain-ends. Second-order kinetic analysis was used to determine the rate constants for uncatalyzed AAC reaction. Electron-deficient alkynes carrying an α-carbonyl undergo a fast Huisgen reaction within a few hours without any catalysts, proportional to the temperature. Less electron-deficient alkynes led to the decrease of AAC reaction rate significantly, revealing that the AAC reaction rate depends on the molecular structure of alkynes center

Similar content being viewed by others
References
W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 28, 15 (2007).
H. C. Kolb, M. G. Finn, and B. Sharpless, Angew. Chem. Int. Ed., 113, 2056 (2001).
H. C. Kolb, M. G. Finn, and B. Sharpless, Angew. Chem. Int. Ed., 40, 2004 (2001).
C. J. Hawker and K. L. Wooley, Science, 309, 1200 (2005).
D. Fournier, R. Hoogenboom, and U. S. Schubert, Chem. Soc. Rev., 36, 1369 (2007).
V. D.Bock, H. Hiemstra, and J. H. van Maarseveen, Eur. J. Org. Chem., 1, 51 (2006).
H. C. Kolb and K. B. Sharpless, Drug Deliv. Today, 8, 1128 (2003).
R. Alvarez, S. Velazquez, A. San-Felix, S. Aquaro, E. De Clereg, C. F. Perno, A. Karlsson, J. Balzarini, and M. J. Camarasa, J. Med. Chem., 37, 4185 (1994).
M. Malkoch, R. Vestberg, N. Gupta, L. Mespouille, P. Dubois, A. F. Mason, J. L. Hedrick, Q. Liao, C. W. Frank, K. Kingbury, and C. J. Hawker, Chem. Commun., 2774 (2006).
Q. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless, and M. G. Finn, J. Am. Chem. Soc., 125, 3192 (2003).
J. Gierlich, G. A. Burley, P. M. E. Gramlich, D. M. Hammond, and T. Carell, Org. Lett., 8, 3639 (2006).
S. T. Laughlin, J. M. Baskin, S. L. Amacher, and C. R. Bertozzi, Science, 320, 664 (2008).
J. A. Johnson, J. M. Baskin, C. R. Bertozzi, J. F.Koberstein, and N. J. Turro, Chem. Commun., 3064 (2008).
J. A. Codelli, J. M. Baskin, N. J. Agard, and C. R. Bertozzi, J. Am. Chem. Soc., 130, 11486 (2008).
E. M. Sletten and C. R. Bertozzi, Org. Lett., 10, 3097 (2008).
J. M. Baskin and C. R. Bertozzi, QSAR Comb. Sci., 26, 1211 (2007).
D. Vuluga, J. Legros, B. Crousse, and D. B. Delpon, Green Chem., 11, 156 (2009).
N. A. Mcgrath and R. T. Raines, Chem. Sci., 3, 3237 (2012).
S. Borukhova, T. Noël, B. Metten, E. de Vos, and V. Hessel, ChemSusChem, 6, 2220 (2013).
C. R. Becer, R. Hoogenboom, and U. S. Schubert, Angew. Chem. Int. Ed., 48, 2 (2009).
S. Sawoo, P. Dutta, A. Chakraborty, R. Mukhopadhyay, O. Bouloussa, and A. Sarkar, Chem. Commun., 5957 (2008).
S. S. van Berkel, A. J. Dirks, S. A. Meeuwissen, D. L. L. Pingen, O. C. Boerman, P. Laverman, F. L. van Delft, J. J. L. Cornelissen, and F. P. J. Rutjes, Chem BioChem, 9, 1805 (2008).
F. Shi, J. P. Waldo, Y. Chen, and R. C. Larock, Org. Lett., 10, 2409 (2008).
J. Hong, Q. Luo, and B. K. Shah, Biomacromolecules, 11, 2960 (2010).
D. Wang, N. Li, M. Zhao, W. Shi, C. Ma, and B. Chen, Green Chem., 12, 2120 (2010).
H. Kang, H. J. Lee, J. C. Park, H. Song, and K. H. Park, Top. Catal., 53, 523 (2010).
R. Thorwirth, A. Stolle, B. Ondruschka, A. Wild, and U. S. Schubert, Chem. Commun., 47, 4370 (2011).
R. Banerjee, S. Maiti, and D. Dhara, Green Chem., 16, 1365 (2014).
T. Keicher, W. Kuglstatter, S. Eisele, T. Wetzel, and H. Krause, Propellants, Explos. Pyrotech., 34, 210 (2009).
B. S. Min, Y. C. Park, and J. C. Yoo, Propellants, Explos. Pyrotech., 37, 59 (2012).
S. K. Reshmi, K. P. Vijayalakshmi, D. Thomas, E. Arunan, and C. P. R. Nair, Propellants, Explos. Pyrotech., 38, 525 (2013).
B. S. Min, Y. C. Park, and J. C. Yoo, Macromol. Res., 20, 429 (2012).
D. H. Lee, K. T. Kim, Y. Jang, S. Lee, H. B. Jeon, H. Paik, B. S. Min, and W. Kim, J. Appl. Polym. Sci., 15, 131 (2014).
A. Omrani, L. C. Simon, A. A. Rostami, and M. Ghaemy, Int. J. Chem. Kinet., 40, 663 (2008).
B. S. Min, H. B. Jeon, T. W. Jeong, and S. Y. Kim, Polym. Chem., 6, 7914 (2015).
R. Huisgen, J. Org. Chem., 33, 2291 (1968).
K. N. Houk, J. Sims, R. E. Duke, R. W. Strozier, and J. K. George, J. Am. Chem. Soc., 95, 7287 (1973).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Min, B.S., Kim, S.Y. Kinetics of in situ robust chain-ends crosslinked polymeric networks formed using catalyst- and solvent-free Huisgen cycloaddition reaction. Macromol. Res. 25, 249–254 (2017). https://doi.org/10.1007/s13233-017-5038-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-017-5038-4