Abstract
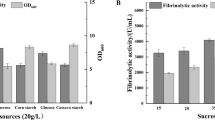
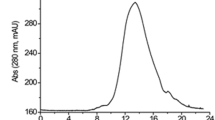
The capacity of fibrinolytic enzymes to degrade blood clots makes them of high relevance in medicine and in the pharmaceutical industry. In this work, forty-three microorganisms of the genus Bacillus were evaluated for their potential to produce fibrinolytic proteases. Thirty bacteria were confirmed as producers of fibrinolytic enzymes, the best results obtained for the strain Bacillus amyloliquefaciens UFPEDA 485. The optimization of the enzyme production conditions was done by a central composite design (CCD) star 23 that allowed to define the optimal conditions for soybean flour and glucose concentrations and agitation rate. The highest fibrinolytic activity (FA) of 813 U mL–1 and a degradation of blood clot in vitro of 62% were obtained in a medium with 2% (w/v) of soybean flour and 1% (w/v) glucose at 200 rpm after 48 h of cultivation, at pH 7.2 and 37 °C. The obtained fibrinolytic enzyme was characterized biochemically. Fibrinolytic activity was inhibited by PMSF (fluoride methylphenylsulfonyl - C7H7FO2S) 91.52% and EDTA (ethylenediaminetetraacetic acid - C10H16N2O8) 89.4%, confirming to be a serine-metallo protease. The optimum pH and temperature were 7.0 and 37 oC, respectively, and the enzyme was stable for 12 h. The fibrinolytic activity at physiological conditions of this enzyme produced by Bacillus amyloliquefaciens UFPEDA 485, as well as its long term stability, demonstrate that it has suitable characteristics for human and veterinary applications, and promises to be a powerful drug for the treatment of vascular diseases.

Similar content being viewed by others
References
M. Schallmey, A. Singh, and O. P. Ward, Can. J. Microbiol., 50, 1 (2004).
P. Rathakrishnan and P. Nagarajan, Int. J. ChemTech Res., 3, 1526 (2011).
S.-H. Choi, K.-S. Whang, J.-S. Park, W.-Y. Choi, and M-H. Yoon, Macromol. Res., 13, 339 (2005).
T. K. Mukhopadhyay, N. Allison, S. Charlton, M. J. Hudson, B. Hallis, A. King, R. Baker, S. Noonan, J. McGlashan, K. West, M. S. Levy, J. M. Ward, and G. J. Lye, Biochem. Eng. J., 50, 139 (2010).
C. D. Sumi, B. W. Yang, I.-C. Yeo, and Y. T. Hahm, Can. J. Microbiol., 61, 93 (2015).
R. Deepak and R. Jayapradha, J. Mycol. Med., 25, 15 (2015).
A. E. Sales, F. A. S. D. Souza, J. A. Teixeira, T. S. Porto, and A. L. F. Porto, Appl. Biochem. Biotechnol., 170, 1676 (2013).
D. N. A Huy, P. A. Hao, and P. V. Hung, Int. Food Res. J., 23, 326 (2016).
S. Sundararajan, C. N. Kannan, and S. Chittibabu, J. Biosci. Bioeng., 111, 128 (2011).
M. Jin, W. Chen, W. Huang, L. Rong, and Z. Gao, Acta Pharm. Sin. B, 3, 123 (2013).
J.-H. Choi, K. Sapkota, S.-E. Park, S. Kim, and S.-J. Kim, Biochimie, 95, 1266 (2013).
E. F. Al-Juamily and B. H. Al-Zaidy, Chem. Sci. Rev. Lett., 2, 256 (2013).
Y. Uesugi, H. Usuki, M. Iwabuchi, and T. Hatanaka, Enzyme Microb. Technol., 48, 7 (2011).
D. N. Avhad, S. S. Vanjari, and V. K. Rathod. Am. J. Curr. Microbiol., 1, 1 (2013).
M. Y. Ahn, B. S. Hahn, K. S. Ryu, J. W. Kim, I. Kim, and Y. S. Kim, Thromb. Res., 112, 339 (2003).
J. He, S. Chen, and J. Gu, FEBS Lett., 518, 2965 (2007).
J. Z. Klafke, M. A. Silva, M. F. Rossato, G. Trevisan, C. I. B. Walker, C. A. M. Leal, D. O. Borges, M. R. C. Schetinger, R. N. Moresco, M. M. M. F. Duarte, A. R. S. Santos, P. R. N. Viecili, and J. Ferreira, Evid.-Based Complement. Alternat. Med., 2012, 1 (2012).
D. W. Kim, K. Sapkota, J. H. Choi, Y. S. Kim, S. Kim, and S. J. Kim, Process Biochem., 48, 340 (2013).
K. Omura, M. Hitosugi, X. Zhu, M. Ikeda, H. Maeda, and S. Tokudome, J. Pharmacol. Sci., 99, 247, (2005).
R. Agrebi, N. Hmidet, M. Hajji, N. Ktari, A. Haddar, N. Fakhfakhzouari, and M. Nasri, Appl. Biochem. Biotechnol., 162, 75 (2010).
A. K. Mukherjee, S. K. Rai, R. Thakur, P. Chattopadhyay, and S. K. Kar, Biochimie, 94, 1300 (2012).
B. K. Bajaj, N. Sharma, and S. Singh, Biocatal. Agric. Biotechnol., 2, 204 (2013).
V. Kanagasabai and V. Thangavelu, J. Adv. Sci. Res., 4, 13 (2013).
A. L. F. Porto, G. M. Campos-Takaki, and J. L. Lima Filho, Appl. Biochem. Biotechnol., 60, 115 (1996).
M. M. Bradford, Anal. Biochem., 72, 248 (1976).
B. Wu, L. Wu, L. Ruan, M. Ge, and D. Chen, Curr. Microbiol., 58, 522 (2009).
T. Astrup and S. Mullertz, Arch. Biochem. Biophys., 40, 346 (1952).
S. Wang, Y. Wu, and T. Liang. N. Biotechnol., 28, 196 (2011).
B. Chen, J. Huo, Z. He, Q. He, Y. Hao, and Z. Chen. Afr. J. Microbiol. Res., 7, 2001 (2013).
A. K. Mukherjee and S. K. Rai, N. Biotechnol., 28, 182 (2011).
J. K. Ki, W. Zhang, and P. Y. Qian, J. Microbiol. Methods, 77, 48 (2009).
B. K. Bajaj, S. Singh, M. Khullar, K. Singh and S. Bhardwaj, Braz. Arch. Biol. Technol., 57, 653 (2014).
G. R. Gad, S. Nirmala, and S. Narendar. Int. J. Pharm. Pharm. Sci., 6, 370 (2014).
P. Vijayaraghavan and S. G. P. Vincent. BioMed Res. Int., 2014, 1 (2014).
V. Mohanasrinivasan, C. S. Devi, R. Biswas, F. Paul, M. Mitra, E. Selvarajan, and V. Suganthi, Bangladesh J. Pharmacol., 8, 110 (2013).
K. Heo, K. M. Cho, C. K. Lee, G. M. Kim, J. H. Shin, J. S. Kim, and J. H. Kim, J. Microbiol. Biotechnol., 23, 974 (2013).
P. M. Mahajan, S. Nayak, and S. S. Lele, J. Biosci. Bioeng., 113, 307 (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, F.A.S.D., Sales, A.E., Costa e Silva, P.E. et al. Optimization of production, biochemical characterization and in vitro evaluation of the therapeutic potential of fibrinolytic enzymes from a new Bacillus amyloliquefaciens . Macromol. Res. 24, 587–595 (2016). https://doi.org/10.1007/s13233-016-4089-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-016-4089-2