Abstract
Background
Congenital Adrenal Hyperplasia (CAH) is a disorder—an ideal candidate to deserve newborn screening. CAH accounts for a significant mortality and morbidity in India, and its awareness among obstetricians should be treated as highly important to prevent the problem.
Purpose of the Study
It is very important for a country like India as the incidence of CAH is reasonably high justifying screening program. However, there are simple logistics that need to be followed, and the treating physicians need to be aware of, if one has to reduce the number of false positives and recalls.
Methods
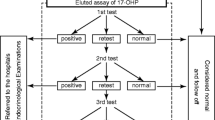
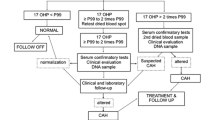
This article takes one through the steps involved in the analysis, interpretation, and reasons for false positives, why the false positives, so that unnecessary calls to parents for repeat sampling are minimized along with the emphasis and the need for the routine screening for CAH.
Results/Conclusion
The results of samples can vary depending on the gestational age of the baby, weight of the baby, sampling time, and the knowledge of these data to the treating Obstetrician and Pediatrician is of paramount importance in preventing repeat samples and frustration for the family and the people involved.
Similar content being viewed by others
References
Lloyd-Puryear MA, Tonniges T, van Dyck PC, et al. American Academy of Pediatrics Newborn Screening Task Force recommendations: how far have we come? Pediatrics. 2006;117(5 Pt.2):S194–211.
Recommended newborn screening tests: 29 disorders—March of Dimes. http://www.marchofdimes.com/professionals/14332_15455.asp.
Wilson JMG, Jungner F. Principles and practice of screening for disease. Public Health Papers No. 34, Geneva: World Health Organization; 1968.
Devi AR, Nausahd SM. Newborn screening in India. Indian J Pediatr. 2004;71(2):157–60.
Kaur G, Srivastav J, Jain S, Chawla D, Chavan BS, Atwal R, Randhawa G, Kaur A, Prasad R. Preliminary report on neonatal screening for congenital hypothyroidism, congenital adrenal hyperplasia and glucose-6-phosphate dehydrogenase deficiency: a Chandigarh experience. Indian J Pediatr. 2010;77:969–73.
Kapoor S, Kabra M. Newborn screening in India: current perspectives. Ind Pediatr. 2010;47(3):219–24.
Verma IC, Bijarnia-Mahay S, Jhingan G, Verma J. Newborn screening need of the hour in India. Indian J Pediatr. 2015;82(1):61–70.
Hiraki S, Green NS. Newborn screening for treatable genetic conditions: past, present and future. Obstet Gynecol Clin N Am. 2010;37:11–21.
American College of Obstetricians and Gynecologists. ACOG committee opinion no. 393, December 2007. Newborn Screening. Obstet Gynecol. 2007; 110.
Padilla CD, Therell BL. Newborn screening in the Asia Pacific region. J Inherit Metab Dis. 2007;30:490–506.
Maiti A, Chatterjee S. Congenital adrenal hyperplasia: an Indian experience. J Paed Child Health. 2011;47(12):883–7.
Van der Kamp HJ, Wit JM. Neonatal screening for congenital adrenal hyperplasia. Eur J Endocrinol. 2004;151(Suppl 3):U71–5.
Pang S. Newborn screening for congenital adrenal hyperplasia. Pediatr Ann. 2003;32(8):516–23.
Pang S, Shook MK. Current status of neonatal screening for congenital adrenal hyperplasia. Curr Opin Pediatr. 1997;9(4):419–23.
Wilcken B. Ethical issues in newborn screening and the impact of new technologies. Eur J Pediatr N Engl J Med. 2003;162(Suppl 1):S62–6.
Warne GL, Armstrong KL, Faunce TA, Wilcken BM, Boneh A, Geelhoed E, Craig ME. The case for newborn screening for congenital adrenal hyperplasia in Australia. Med J Aust. 2010;192(2):107.
Olgemoller B, Roscher AA, Liebel B, Fingerhut R. Screening for congenital adrenal hyperplasia: adjustment of 17-hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J Clin Endocrinol Metab. 2003;88:5790–4.
Tajima T, Fujikura K, Fukushi M, Hotsubo T, Mitsuhashi Y. Neonatal screening for congenital adrenal hyperplasia in Japan. Pediatr Endocrinol Rev. 2012;10(Suppl 1):72–8.
Choudhuri T, Sengupta S. Inborn errors of metabolism—an Indian perspective. Int J Hum Genet. 2006;6:89–91.
Committee on Genetics. Issues in newborn screening. Paediatrics. 1992;89:345–9.
Verma IC, Bijarnia S. The burden of genetic disorders in India and a framework for community control. Community Genet. 2002;5:192–6.
Cunningham G. The science and politics of screening newborns. N Engl J Med. 2002;346(14):1084–5.
Nagar N, Kishore Kumar R. Challenges in implementation of universal neonatal screening in India: P56—Poster presentation @ NNF, Pune 13–16 December 2007.
Menon PSN, Virmani A, Sethi AK, Verma IC, Rohatgi M, Gupta DK, Gupta AK. Congenital adrenal hyperplasia: experience at intersex clinic, AIIMS. Indian J Pediatr. 1992;59:531–5.
Fernhoff PM. Newborn screening for genetic disorders. Pediatr Clin N Am. 2009;56(3):505–13.
Compliance with Ethical Requirements and Conflict of Interest
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. R. Kishore Kumar is a Consultant Neonatologist & Paediatrician in Cloudnine Hospitals and Adjunct Professor in Neonatology & Paediatrics, Notre Dame University; Dr. Hari Das is the Chief Pathologist in Cloudnine Hospitals; Dr. Prakash Kini is a Senior Consultant Obstetrician & Gynaecologist in Cloudnine Hospitals.
Rights and permissions
About this article
Cite this article
Kishore Kumar, R., Das, H. & Kini, P. Newborn Screening for Congenital Adrenal Hyperplasia in India: What Do We Need to Watch Out for?. J Obstet Gynecol India 66, 415–419 (2016). https://doi.org/10.1007/s13224-015-0712-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-015-0712-y