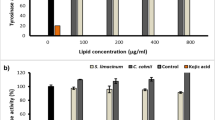
Abstract
Tyrosinase is the main enzyme responsible for enzymatic browning of fruits post-harvest and melanogenesis in mammals, an undesirable phenomenon. This encouraged researchers to seek potent tyrosinase inhibitors for application in the food and cosmetics industries. Despite an increased knowledge of tyrosinase inhibitors from plants and synthetic sources in the past few years, inhibitors of microbial origin are under-explored. Thus, this article surveys tyrosinase inhibitors produced by microorganisms and hence, serves as an updated database of tyrosinase inhibitors from microbial sources.





Similar content being viewed by others
References
Abraham K, Gürtler R, Berg K, Heinemeyer G, Lampen A, Appel KE (2011) Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. Mol Nutr Food Res 55:667–678. doi:10.1002/mnfr.201000564
Arai N, Shiomi K, Takamatsu S, Komiyama K, Shinose M, Takahashi Y, Tanaka Y, Iwai Y, Liu JR, Omura S (1997) Amphistin, a new melanogenesis inhibitor, produced by an actinomycete. J Antibiot 50(10):808–814
Bajpai VK, Rather IA, Park YH (2016) Partially purified exo-polysaccharide from Lactobacillus sakei Probio 65 with antioxidant, α-glucosidase and tyrosinase inhibitory potential. J Food Biochem 40(3):264–274. doi:10.1111/jfbc.12230
Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, Ballotti R (1998) Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol 142:827–835
Borges CR, Roberts JC, Wilkins DG, Rollins DE (2001) Relationship of melanin degradation products to actual melanin content: application to human hair. Anal Biochem 290:116–125
Brenner M, Hearing VJ (2008) The protective role of melanin against UV damage in human skin. Photochem Photobiol 84(3):539–549
Burdock GA, Soni MG, Carabin IG (2001) Evaluation of health aspects of kojic acid in food. Regul Toxicol Pharmacol 33(1):80–101
Busch JM (1999) Enzymic browning in potatoes: a simple assay for a polyphenol oxidase catalysed reaction. Biochem Educ 27:171–173
Cabrera-Valladares N, Martínez A, Piñero S, Lagunas-Munoz VH, Tinoco R, De Anda R, Vázquez-Duhalt R, Bolívar F, Gosset G (2006) Expression of the melA gene from Rhizobium etli CFN42 in Escherichia coli and characterization of the encoded tyrosinase. Enzym Microb Technol 38:772–779
Capuano E, Fogliano V (2011) Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. Food Sci Technol 44:793–810
Cerenius L, Söderhäll K (2004) The prophenoloxidase-activating system in invertebrates. Immunol Rev 198:116–126
Chan CF, Huang CC, Lee MY, Lin YS (2014) Fermented broth in tyrosinase and melanogenesis inhibition. Molecules 19:13122–13135
Chang TS (2009) An updated review of tyrosinase inhibitors. Int J Mol Sci 10:2440–2475
Chang TS (2012a) Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 5:1661–1685
Chang TM (2012b) Tyrosinase and tyrosinase inhibitors. J Biocatal Biotransfor 1:2
Chang CJ, Tsai TY (2016) Antimelanogenic effects of the novel melanogenic inhibitors daidzein and equol, derived from soymilk fermented with Lactobacillus plantarum strain TWK10, in B16F0 mouse melanoma cells. J Funct Foods 22:211–223
Chang TS, Tseng M (2006) Preliminary screening of soil actinomycetes for anti-tyrosinase activity. J Mar Sci Technol 14(3):190–193
Chang TS, Ding HY, Tai SS, Wu CY (2007) Mushroom tyrosinase inhibitory effects of isoflavones isolated from soygerm koji fermented with Aspergillus oryzae BCRC 32288. Food Chem 105:1430–1438
Chang TS, Tseng M, Ding HY, Tai SS (2008) Isolation and characterization of Streptomyces hiroshimensis strain TI-C3 with anti-tyrosinase activity. J Cosmet Sci 59:33–40
Chang CJ, Dai RY, Leu YL, Tsai TY (2015) Effects of the melanogenic inhibitor, uracil, derived from Lactobacillus plantarum TWK10-fermented soy milk on anti-melanogenesis in B16F0 mouse melanoma cells. J Funct Foods 17:314–327
Chen YM, Shih TW, Chiu CP, Pan TM, Tsai TY (2013) Effects of lactic acid bacteria-fermented soy milk on melanogenesis in B16F0 melanocytes. J Funct Foods 5:395–405
Chen CY, Lin LC, Yang WF, Bordon J, Wang HMD (2015) An updated organic classification of tyrosinase inhibitors on melanin biosynthesis. Curr Org Chem 19:4–18
Choudhary MI, Sultan S, Khan MTH, Atta-ur-Rahman (2005) Microbial transformation of 17α-ethynyl- and 17α-ethylsteroids, and tyrosinase inhibitory activity of transformed products. Steroids 70:798–802
Claus H, Decker H (2006) Bacterial tyrosinases. Syst Appl Microbiol 29:3–14
Dalfard AB, Khajeh K, Soudi MR, Naderi-Manesh H, Ranjbar B, Sajedi RH (2006) Isolation and biochemical characterization of laccase and tyrosinase activities in a novel melanogenic soil bacterium. Enzym Microb Technol 39:1409–1416
Deering RW, Chen J, Sun J, Ma H, Dubert J, Barja JL, Seeram NP, Wang H, Rowley DC (2016) N-acyl dehydrotyrosines, tyrosinase inhibitors from the marine bacterium Thalassotalea sp. PP2-459. J Nat Prod 79:447–450. doi:10.1021/acs.jnatprod.5b00972
Fritz WA, Coward L, Wang J, Lamartiniere CA (1998) Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis 19(12):2151–2158
Fujii Y, Asahara M, Ichinoe M, Nakajima H (2002) Fungal melanin inhibitor and related compounds from Penicillium decumbens. Phytochemistry 60(7):703–708
García‐Borrón JC, Solano F (2002) Molecular anatomy of tyrosinase and its related proteins: beyond the histidine-bound metal catalytic center. Pigment Cell Res 15:162–173
Goetghebeur M, Kermasha S (1996) Inhibition of polyphenol oxidase by copper-metallothionein from Aspergillus niger. Phytochemistry 42:935–940
Gillbro JM, Olsson MJ (2011) The melanogenesis and mechanisms of skin-lightening agents—existing and new approaches. Int J Cosmet Sci 33:210–221. doi:10.1111/j.1468-2494.2010.00616.x
Goldfeder M, Kanteev M, Isaschar-Ovdat S, Adir N, Fishman A (2014) Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat Commun 5:4505. doi:10.1038/ncomms5505
Halaouli S, Asther M, Kruus K, Guo L, Hamdi M, Sigoillot JC, Asther M, Lomascolo A (2005) Characterization of a new tyrosinase from Pycnoporus species with high potential for food technological applications. J Appl Microbiol 98:332–343
Halaouli S, Asther M, Sigoillot JC, Hamdi M, Lomascolo A (2006) Fungal tyrosinases: new prospects in molecular characteristics, bioengineering and biotechnological applications. J Appl Microbiol 100(2):219–232
Haudecoeur R, Gouron A, Dubois C, Jamet H, Lightbody M, Hardré R, Milet A, Bergantino E, Bubacco L, Belle C, Réglier M, Boumendjel A (2014) Investigation of binding-site homology between mushroom and bacterial tyrosinases by using aurones as effectors. Chembiochem 15(9):1325–1333. doi:10.1002/cbic.201402003
Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE (1998) MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 391:298–301
Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4:988–1004
Heo IS, Kim KS, Yang SY, Lee NH, Lim SD (2007) Physiological characteristics and tyrosinase inhibitory activity of Lactobacillus plantarum M23 isolated from raw milk. Korean J Food Sci Anim Resour 27(4):501–508
Hernández-Romero D, Solano F, Sanchez-Amat A (2005) Polyphenol oxidase activity expression in Ralstonia solanacearum. Appl Environ Microbiol 71(11):6808–6815. doi:10.1128/AEM.71.11.6808-6815.2005
Hernández-Romero D, Sanchez-Amat A, Solano F (2006) A tyrosinase with an abnormally high tyrosine hydroxylase/dopa oxidase ratio. FEBS J 273:257–270
Hsu CH, Nguyen AD, Chen YW, Wang SL (2014) Tyrosinase inhibitors and insecticidal materials produced by Burkholderia cepacia using squid pen as the sole carbon and nitrogen source. Res Chem Intermed 40:2249–2258. doi:10.1007/s11164-014-1602-0
Huang HC, Chang TM (2012) Antioxidative properties and inhibitory effect of Bifidobacterium adolescentis on melanogenesis. World J Microbiol Biotechnol 28(9):2903–2912
Ichishima E, Maeba H, Amikura T, Sakata H (1984) Multiple forms of protyrosinase from Aspergillus oryzae and their mode of activation at pH 3.0. Biochim Biophys Acta 786:25–31
Imada C (2004) Enzyme inhibitors of marine microbial origin with pharmaceutical importance. Mar Biotechnol 6:193–198
Imada C, Sugimoto Y, Makimura T, Kobayashi T, Hamada N, Watanabe E (2001) Isolation and characterization of tyrosinase inhibitor-producing microorganisms from marine environment. Fish Sci 67:1151–1156
Ioannou I, Ghoul M (2013) Prevention of enzymatic browning in fruit and vegetables. Eur Sci J 9(30):310–341
Ishihara Y, Oka M, Tsunakawa M, Tomita K, Hatori M, Yamamoto H, Kamei H, Miyaki T, Konishi M, Oki T (1991) Melanostatin, a new melanin synthesis inhibitor. Production, isolation, chemical properties, structure and biological activity. J Antibiot 44(1):25–32
Kanda K, Sato T, Ishii S, Enei H, Ejiri S (1996) Purification and properties of tyrosinase isozymes from the gill of Lentinus edodes fruiting body. Biosci Biotechnol Biochem 60:1273–1278
Kang HS, Choi JH, Cho WK, Park JC, Choi JS (2004) A sphingolipid and tyrosinase inhibitors from the fruiting body of Phellinus linteus. Arch Pharm Res 27(7):742–750
Kanteev M, Goldfeder M, Fishman A (2015) Structure–function correlations in tyrosinases. Protein Sci 24:1360–1369
Katz E, Thompson CJ, Hopwood DA (1983) Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol 129:2703–2714
Kawagishi H, Somoto A, Kuranari J, Kimura A, Chiba S (1993) A novel cyclotetrapeptide produced by Lactobacillus helveticus as a tyrosinase inhibitor. Tetrahedron Lett 34(21):3439–3440
Khan MTH (2007) Molecular design of tyrosinase inhibitors: a critical review of promising novel inhibitors from synthetic origins. Pure Appl Chem 79(12):2277–2295
Kilimnik A, Dembitsky VM (2016) Anti-melanoma agents derived from fungal species. Mathews J Pharm Sci 1(1):002
Kim YJ, Uyama H (2005) Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci 62:1707–1723
Kim JP, Kim BK, Yun BS, Ryoo IJ, Lee CH, Lee IK, Kim WG, Lee S, Pyun YR, Yoo ID (2003) Melanocins A, B and C, new melanin synthesis inhibitors produced by Eupenicillium shearii I. Taxonomy, fermentation, isolation and biological properties. J Antibiot 56(12):993–999
Kim WG, Ryoo IJ, Park SH, Kim DS, Lee S, Park KC, Yoo ID (2005) Terrein, a melanin biosynthesis inhibitor, from Penicillium sp. 20135. J Microbiol Biotechnol 15(4):891–894
Kim DS, Cho HJ, Lee HK, Lee WH, Park ES, Youn SW, Park KC (2007) Terrein, a fungal metabolite, inhibits the epidermal proliferation of skin equivalents. J Dermatol Sci 46(1):65–68
Kim DS, Lee HK, Park SH, Lee S, Ryoo IJ, Kim WG, Yoo ID, Na JI, Kwon SB, Park KC (2008) Terrein inhibits keratinocyte proliferation via ERK inactivation and G2/M cell cycle arrest. Exp Dermatol 17(4):312–317
Kim HR, Kim H, Jung BJ, You GE, Jang S, Chung DK (2015) Lipoteichoic acid isolated from Lactobacillus plantarum inhibits melanogenesis in B16F10 mouse melanoma cells. Mol Cells 38(2):163–170
Kobayashi T, Vieira WD, Potterf B, Sakai C, Imokawa G (1995) Modulation of melanogenic protein expression during the switch from eu- to pheomelanogenesis. J Cell Sci 108:2301–2309
Kong KH, Hong MP, Choi SS, Kim YT, Cho SH (2000) Purification and characterization of a highly stable tyrosinase from Thermomicrobium roseum. Biotechnol Appl Biochem 31(2):113–118. doi:10.1042/BA19990096
Kuwaki S, Nakajima N, Tanaka H, Ishihara K (2012) Plant-based paste fermented by lactic acid bacteria and yeast: functional analysis and possibility of application to functional foods. Biochem Insights 5:21–29. doi: 10.4137/BCI.S10529
Lamartiniere CA, Wang J, Smith-Johnson M, Eltoum IE (2002) Daidzein: bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol Sci 65:228–238
Lee CH, Chung MC, Lee HJ, Kho YH, Lee KH (1995) MR304-1, a melanin synthesis inhibitor produced by Trichoderma harzianum. Korean J Appl Microbiol Biotechnol 23(6):641–646
Lee CH, Chung MC, Lee HJ, Bae KS, Kho YH (1997a) MR566A and MR566B, new melanin synthesis inhibitors produced by Trichoderma harzianum. I. Taxonomy, fermentation, isolation and biological activities. J Antibiot 50(6):469–473
Lee CH, Koshino H, Chung MC, Lee HJ, Hong JK, Yoon JS, Kho YH (1997b) MR566A and MR566B, new melanin synthesis inhibitors produced by Trichoderma harzianum. II. Physico-chemical properties and structural elucidation. J Antibiot 50(6):474–478
Lerch K (1981) Copper monooxygenases: tyrosinase and dopamine β-monooxygenase. In: Sigel H (ed) Metal ions in biological systems. Marcel Dekker, New York, pp 143–186
Lerch K (1983) Neurospora tyrosinase: structural, spectroscopic and catalytic properties. Mol Cell Biochem 52:125–138
Lerch K, Ettinger L (1972) Purification and characterization of a tyrosinase from Streptomyces glaucescens. Eur J Biochem 31:427–437
Li X, Jeong JH, Lee KT, Rho JR, Choi HD, Kang JS, Son BW (2003) Gamma-pyrone derivatives, kojic acid methyl ethers from a marine-derived fungus Alternaria sp. Arch Pharm Res 26(7):532–534
Li X, Kim MK, Lee U, Kim SK, Kang JS, Choi HD, Son BW (2005) Myrothenones A and B, cyclopentenone derivatives with tyrosinase inhibitory activity from the marine-derived fungus Myrothecium sp. Chem Pharm Bull 53(4):453–455
Liang TW, Lee YC, Wang SL (2015) Tyrosinase inhibitory activity of supernatant and semi-purified extracts from squid pen fermented with Burkholderia cepacia TKU025. Res Chem Intermed 41(9):6105–6116
Likhitwitayawuid K (2008) Stilbenes with tyrosinase inhibitory activity. Curr Sci 94(1):44–52
Lim SD, Kim KS (2012) Optimization of tyrosinase inhibitory activity in the fermented milk by Lactobacillus plantarum M23. Korean J Food Sci Anim Resour 32(5):678–684
Lin JW, Chiang HM, Lin YC, Wen KC (2008) Natural products with skin-whitening effects. J Food Drug Anal 16(2):1–10
Liu N, Zhang T, Wang YJ, Huang YP, Ou JH, Shen P (2004) A heat inducible tyrosinase with distinct properties from Bacillus thuringiensis. Lett Appl Microbiol 39:407–412
Loizzo MR, Tundis R, Menichini F (2012) Natural and synthetic tyrosinase inhibitors as antibrowning agents: an update. Compr Rev Food Sci Food Saf 11:378–398
Lu Q, Tian M, Liu Y, Yu D (2002) Isolation and structure elucidation of melanin biosynthesis inhibitors H7264 A and B. Zhongguo Kangshengsu Zazhi 27(7):385–386
Lu R, Liu X, Gao S, Zhang W, Peng F, Hu F, Huang B, Chen L, Bao G, Li C, Li Z (2014) New tyrosinase inhibitors from Paecilomyces gunnii. J Agric Food Chem 62(49):11917–11923. doi:10.1021/jf504128c
Madhosingh C, Sundberg L (1974) Purification and properties of tyrosinase inhibitor from mushroom. FEBS Lett 49:156–158
Matoba Y, Kumagai T, Yamamoto A, Yoshitsu H, Sugiyama M (2006) Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J Biol Chem 281:8981–8990
Mayer AM (1987) Polyphenol oxidases in plants—recent progress. Phytochemistry 26:11–20
Mayer AM, Harel E (1979) Polyphenol oxidases in plants. Phytochemistry 18:193–215
McMahon AM, Doyle EM, Brooks S, O’Connor KE (2007) Biochemical characterisation of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6. Enzym Microb Technol 40:1435–1441
Meinkoth JL, Montminy MR, Fink JS, Feramisco JR (1991) Induction of a cyclic AMP-responsive gene in living cells requires the nuclear factor CREB. Mol Cell Biol 11:1759–1764
Menon S, Fleck RW, Yong G, Strothkamp KG (1990) Benzoic acid inhibition of the α, β, and γ isozymes of Agaricus bisporus tyrosinase. Arch Biochem Biophys 280:27–32
Michalik J, Emilianowicz-Czerska W, Switalski L, Raczynska-Bojanowska K (1975) Monophenol monooxygenase and lincomysin biosynthesis in Streptomyces lincolnensis. Antimicrob Agents Chemother 8(5):526–531
Minosasa J, Matsui K, Uehara H, Tanaka H (1991) Tyrosinase inhibitors containing neogrifolin. Jpn Kokai Tokkyo Koho, 15 Japanese Patent: JP 03109319 A 19910509 Heisei
Misasa H, Matsui Y, Uehara H, Tanaka H, Ishihara M, Shibata H (1992) Tyrosinase inhibitors from Albatrellus confluens. Biosci Biotechnol Biochem 56(10):1660–1661
Morimura K, Yamazaki C, Hattori Y, Makabe H, Kamo T, Hirota M (2007) A tyrosinase inhibitor, Daedalin A, from mycelial culture of Daedalea dickinsii. Biosci Biotechnol Biochem 71(11):2837–2840
Morimura K, Hiramatsu K, Yamazaki C, Hattori Y, Makabe H, Hirota M (2009) Daedalin A, a metabolite of Daedalea dickinsii, inhibits melanin synthesis in an in vitro human skin model. Biosci Biotechnol Biochem 73(3):627–632. doi:10.1271/bbb.80695
Müller WE, Grebenjuk VA, Thakur NL, Thakur AN, Batel R, Krasko A, Müller IM, Breter HJ (2004) Oxygen-controlled bacterial growth in the sponge Suberites domuncula: toward a molecular understanding of the symbiotic relationships between sponge and bacteria. Appl Environ Microbiol 70:2332–2341
Nakashima T, Anzai K, Kuwahara N, Komaki H, Miyadoh S, Harayama S, Tianero MDB, Tanaka J, Kanamoto A, Ando K (2009) Physicochemical characters of a tyrosinase inhibitor produced by Streptomyces roseolilacinus NBRC 12815. Biol Pharm Bull 32(5):832–836
Nambudiri AMD, Bhat JV, Rao PVS (1972) Conversion of p-coumarate into caffeate by Streptomyces nigrifaciens: purification and properties of the hydroxylating enzyme. Biochem J 130:425–433
Nicolas JJ, Richard-Forget FC, Goupy PM, Amiot MJ, Aubert SY (1994) Enzymatic browning reactions in apple and apple products. Crit Rev Food Sci Nutr 34:109–157
Nohynek GJ, Kirkland D, Marzin D, Toutain H, Leclerc-Ribaud C, Jinnai H (2004) An assessment of the genotoxicity and human health risk of topical use of kojic acid [5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one]. Food Chem Toxicol 42:93–105
Park SH, Kim DS, Kim WG, Ryoo IJ, Lee DH, Huh CH, Youn SW, Yoo ID, Park KC (2004) Terrein: a new melanogenesis inhibitor and its mechanism. Cell Mol Life Sci 61:2878–2885
Parvez S, Kang M, Chung HS, Bae H (2007) Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytother Res 21:805–816
Philipp S, Held T, Kutzner HJ (1991) Purification and characterization of the tyrosinase of Streptomyces michiganensis DSM 40015. J Basic Microbiol 31:293–300
Pomerantz SH, Murthy VV (1974) Purification and properties of tyrosinases from Vibrio tyrosinaticus. Arch Biochem Biophys 160(1):73–82
Raistrick H, Smith G (1935) Studies in the biochemistry of micro-organisms: the metabolic products of Aspergillus terreus Thom. A new mould metabolic product—terrein. Biochem J 29(3):606–611
Raper HS (1928) The anaerobic oxidases. Physiol Rev 8:245–282
Razak DLA, Rashid NYA, Jamaluddin A, Sharifudin SA, Kahar AA, Long K (2015) Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J Saudi Soc Agric Sci. doi:10.1016/j.jssas.2015.04.001
Robb DA (1984) Tyrosinase. In: Lontie R (ed) Copper proteins and copper enzymes, vol 2. CRC Press, Boca Raton, pp 207–241
Ruan L, He W, He J, Sun M, Yu Z (2005) Cloning and expression of mel gene from Bacillus thuringiensis in Escherichia coli. Antonie van Leeuwenhoek 87:283–288
Sánchez-Ferrer Á, Rodríguez-López JN, García-Cánovas F, García-Carmona F (1995) Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta 1247:1–11
Saruno R, Kato F, Ikeno T (1979) Kojic acid, a tyrosinase inhibitor from Aspergillus albus. Agric Biol Chem 43(6):1337–1338
Schaffer JV, Bolognia JL (2001) The melanocortin-1 receptor: red hair and beyond. Arch Dermatol 137:1477–1485
Schallreuter KU, Wood JW (1990) A possible mechanism of action for azelaic acid in the human epidermis. Arch Dermatol Res 282:168–171
Schurink M, van Berkel WJH, Wichers HJ, Boeriu CG (2007) Novel peptides with tyrosinase inhibitory activity. Peptides 28:485–495
Seiberg M, Paine C, Sharlow E, Eisinger M, Shapiro SS, Andrade-Gordon P, Costanzo M (2000) Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol 115:162–167
Selinheimo E, Saloheimo M, Ahola E, Westerholm-Parvinen A, Kalkkinen N, Buchert J, Kruus K (2006) Production and characterization of a secreted, C-terminally processed tyrosinase from the filamentous fungus Trichoderma reesei. FEBS J 273:4322–4335
Selinheimo E, Nieidhin D, Steffensen C, Nielsen J, Lomascolo A, Halaouli S, Record E, O’Beirne D, Buchert J, Kruus K (2007) Comparison of the characteristics of fungal and plant tyrosinases. J Biotechnol 130:471–480
Sharma VK, Choi J, Sharma N, Choi M, Seo SY (2004) In vitro anti-tyrosinase activity of 5-(hydroxymethyl)-2-furfural isolated from Dictyophora indusiata. Phytother Res 18(10):841–844
Shuster V, Fishman A (2009) Isolation, cloning and characterization of a tyrosinase with improved activity in organic solvents from Bacillus megaterium. J Mol Microbiol Biotechnol 17:188–200
Singh P, Langowski HC, Wani AA, Saengerlaub S (2010) Recent advances in extending the shelf life of fresh Agaricus mushrooms: a review. J Sci Food Agric 90:1393–1402
Solano F, Briganti S, Picardo M, Ghanem G (2006) Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res 19:550–571. doi:10.1111/j.1600-0749.2006.00334.x
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96:2563–2605
Sugumaran M (2002) Comparative biochemistry of Eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res 15:2–9
Takahashi S, Iwai H, Kosaka K, Miyazaki T, Osanai Y, Arao N, Tanaka K, Nagai K, Nakagawa A (2007) Byelyankacin: a novel melanogenesis inhibitor produced by Enterobacter sp. B20. J Antibiot 60(11):717–720
Takamatsu S, Rho MC, Hayashi M, Komiyama K, Tanaka H, Omura S, Imokawa G (1993) New inhibitors of melanogenesis, OH-3984 K1 and K2. II. Physico-chemical properties and structural elucidation. J Antibiot 46(10):1526–1529
Takamatsu S, Kim YP, Hayashi M, Komiyama K, Imokawa G, Omura S (1996) A new inhibitor of melanogenesis, Albocycline K3, produced by Streptomyces sp. OH-3984. J Antibiot 49(5):485–486
Tanaka N, Naganuma M, Fukuda M, Wati Y, Komatsu K, Yoshida S, Komiyama K, Omura S (1996) Novel inhibitor of melanogenesis produced by Talaromyces FO-3182. Nippon Koshohin Kagakkaishi 20(1):3–6
Töpert M, Rach P, Siegmund F (1989) Pharmacology and toxicology of azelaic acid. Acta Derm Venereol Suppl 143:14–19
Tsai CC, Chan CF, Huang WY, Lin JS, Chan P, Liu HY, Lin YS (2013) Applications of Lactobacillus rhamnosus spent culture supernatant in cosmetic antioxidation, whitening and moisture retention applications. Molecules 18:14161–14171. doi:10.3390/molecules181114161
Tsuchiya T, Yamada K, Minoura K, Miyamoto K, Usami Y, Kobayashi T, Hamada-Sato N, Imada C, Tsujibo H (2008) Purification and determination of the chemical structure of the tyrosinase inhibitor produced by Trichoderma viride strain H1-7 from a marine environment. Biol Pharm Bull 31(8):1618–1620
Umezawa H (1972) Enzyme inhibitors of microbial origin. University of Tokyo Press, Tokyo, Japan
Van Gelder CW, Flurkey WH, Wichers HJ (1997) Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 45:1309–1323
Vasantha KY, Murugesh CS, Sattur AP (2014) A tyrosinase inhibitor from Aspergillus niger. J Food Sci Technol 51(10):2877–2880
Walker JRL, McCallion RF (1980) The selective inhibition of ortho- and para-diphenol oxidases. Phytochemistry 19:373–377
Wang GH, Chen CY, Lin CP, Huang CL, Lin CH, Cheng CY, Chung YC (2016) Tyrosinase inhibitory and antioxidant activities of three Bifidobacterium bifidum-fermented herb extracts. Ind Crop Prod 89:376–382
Wei CI, Huang TS, Chen JS, Marshall MR, Chung KT (1991) Production of kojic acid by Aspergillus candidus in three culture media. J Food Prot 54(7):546–548
Wu B, Wu X, Sun M, Li M (2013) Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233. Mar Drugs 11:2713–2721
Xu W, Gong L, Haddad MM, Bischof O, Campisi J, Yeh ET, Medrano EE (2000) Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp Cell Res 255:135–143
Yoshida H, Tanaka Y, Nakayama K (1974) Properties of tyrosinase from Pseudomonas melanogenum. Agric Biol Chem 38(3):627–632
Yoshimoto T, Yamamoto K, Tsuru D (1985) Extracellular tyrosinase from Streptomyces sp. KY-453: purification and some enzymatic properties. J Biochem 97(6):1747–1754
Zhang D, Li X, Kang JS, Choi HD, Son BW (2007) A new a-pyrone derivative, 6-[(E)-hept-1-enyl]-a-pyrone, with tyrosinase inhibitory activity from a marine isolate of the fungus Botrytis. Bull Kor Chem Soc 28(5):887–888
Zheng ZP, Cheng KW, Chao J, Wu J, Wang M (2008) Tyrosinase inhibitors from paper mulberry (Broussonetia papyrifera). Food Chem 106:529–535
Zhuravleva OI, Afiyatullov SS, Vishchuk OS, Denisenko VA, Slinkina NN, Smetanina OF (2012) Decumbenone C, a new cytotoxic decaline derivative from the marine fungus Aspergillus sulphureus KMM 4640. Arch Pharm Res 35(10):1757–1762
Acknowledgements
The authors wish to thank the Head of the Department of Biotechnology for the facilities provided and UGC-MANF scholarship for the funds provided (MANF-2012-13-CHR-GOA-12673 to M.S.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the UGC-Maulana Azad fellowship (MANF-2012-13-CHR-GOA-12673 to M.S.F.).
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Fernandes, M.S., Kerkar, S. Microorganisms as a source of tyrosinase inhibitors: a review. Ann Microbiol 67, 343–358 (2017). https://doi.org/10.1007/s13213-017-1261-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-017-1261-7