Abstract
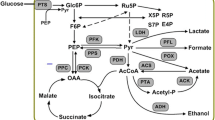
The production of L-tryptophan was increased by reducing acetate accumulation through a decrease in acetate kinase activity by gene deletion. The effects of disruption of the genes for acetate kinase (ackA) and an enzyme with propionate/acetate kinase activity (tdcD) on L-tryptophan production were investigated. The ackA and/or tdcD deletion mutants accumulated less acetate and more L-tryptophan than the parental strain. Furthermore, the production of L-tryptophan obtained with ackA-tdcD mutant were more than the mutants with a single deletion of ackA or tdcD, while higher production of L-tryptophan and lower concentration of acetate were accumulated in the ackA mutant than the mutant with a lesion in tdcD. In L-tryptophan fed-batch fermentation using the ackA-tdcD mutant, the excretion of acetate was reduced to 1.22 g/L, a 21.79 % reduction compared with the parental strain, and the production of L-tryptophan and glucose conversion rate were increased to 52.5 g/L and 47.9 g/L, respectively, which represented 6.49 % and 10.88 % increases compared with the parental strain, and the glucose conversion rate reached a high level of 21.2 %, which was 8.16 % higher than the parental strain. In addition, the metabolic flux analysis of TRTH and TRTHAT indicated that the carbon flux through EMP was decreased by 8.37 % and the carbon flux through PP was increased by 57.03 % in TRTHAT compared with TRTH. The flux of acetate and tryptophan formation of TRTHAT were 5.2 % and 17.3 %, which were 3.67-times lower and 1.75-times higher than these of TRTH, respectively.






Similar content being viewed by others
References
Báez-Viveros JL, Flores N, Juárez K, Castillo-España P, Bolivar F, Gosset G (2007) Metabolic transcription analysis of engineered Escherichia coli strains that overproduce L-phenylalanine. Microb Cell Factories. doi:10.1186/1475-2859-6-30
Castaño-Cerezo S, Pastor JM, Renilla S, Bernal V, Iborra JL, Cánovas M (2009) An insight into the role of phosphotransacetylase (pta) and the acetate/acetyl-CoA node in Escherichia coli. Microb Cell Factories. doi:10.1186/1475-2859-8-54
Chang DE, Shin S, Rhee JS, Pan JG (1999) Acetate metabolism in a pta mutant of Escherichia coli W3110: importance of maintaining acetyl coenzyme a flux for growth and survival. J Bacteriol 6656-6663
Cheng LK, Wang J, Xu QY, Xie XX, Zhang YJ, Zhao CG, Chen N (2012) Effect of feeding strategy on L-tryptophan production by recombinant Escherichia coli. Ann Microbiol 62:1625–1634
Cherepanov PP, Wackernagel W (1995) Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158(1):9–14
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97(12):6640–6645
De Anda R, Lara AR, Hernandez V, Hernandez-Montalvoa V, Gosset G, Bolıvar F, Ramırez OT (2006) Replacement of the glucose phosphotransferase transport system by galactose permease reduces acetate accumulation and improves process performance of Escherichia coli for recombinant protein production without impairment of growth rate. Metab Eng 8:281–290
De Mey M, Lequeux GJ, Beauprez JJ, Maertens J, Horen EV, Soetaert WK, Vanrolleghem PA, Vandamme EJ (2007) Comparison of different strategies to reduce acetate formation in Escherichia coli. Biotechnol Prog 23:1053–1063
Edwards JS, Palsson (2000) Metabolic flux balance analysis and the in silico analysis of Escherichia coli K-12 gene deletion. BMC Bioinformatics 1:1
Eiteman MA, Altman E (2006) Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol 24(11):530–536
Flores S, Gosset G, Flores N, De Graff AA, Bolivar F (2002) Analysis of carbon metabolism in Escherichia coli strains with an inactive phosphotransferase system by 13C labeling and NMR spectroscopy. Metab Eng 4:124–137
Gosset G (2005) Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: sugar phosphotransferase system. Microb Cell Factories. doi:10.1186/1475-2859-4-14
Grundy FJ, Waters DA, Allen SH, Henkin TM (1993) Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J Bacteriol 175:7348–7355
Gu PF, Yang F, Kang JH, Wang Q, Qi QS (2012) One-step of tryptophan attenuator inactivation and promoter swapping to improve the production of L-tryptophan in Escherichia coli. Microb Cell Factories 11:30
Hajji M, Kanoun S, Nasri M, Gharsallah N (2007) Purification and characterization of an alkaline serine-protease produced by a new isolated Aspergillus clavatus ES1. Process Biochem 42:791–797
Heßlinger G, Fairhurst SA, Sawers G (1998) Novel keto acid formate-lyase and propionate kinase enzymes are components of an anaerobic pathway in Escherichia coli that degrades L-threonine to propionate. Mol Microbiol 27(2):477–492
Huang J, Shi JM, Liu Q, Xu QY, Xie XX, Wen TY, Chen N (2011) Effects of gene pta disruption on L-tryptophan fermentation. Acta Microbiol Sin 51(4):480–487
Ikeda M (2006) Towards bacterial strains overproducing L-tryptophan and other aromatics by metabolic engineering. Appl Microbiol Biotechnol 69:615–626
Jantama K, Zhang XL, Moore JC, Shanmugam KT, Svoronos SA, Ingram LO (2008) Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol Bioeng 101(5):881–893
Kim JYH, Cha HJ (2003) Down-regulation of acetate pathway through antisense strategy in Escherichia coli: improved foreign protein production. Biotechnol Bioeng 83:841–853
Kumari S, Tishel R, Eisenbach M, Wolfe AJ (1995) Cloning, characterization and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol 177:2878–2886
Liu Q, Cheng YS, Xie XX, Xu QY, Chen N (2012) Modification of tryptophan transport system and its impact on production of L-tryptophan in Escherichia coli. Bioresour Technol 114:549–554
Martínez-Gómez K, Flores N, Castañeda HM, Martínez-Batallar G, Hernández-Chávez G, Ramírez OT, Gosset G, Encarnación S, Bolivar F (2012) New insights into Escherichia coli metabolism: carbon scavenging, acetate metabolism and carbon recycling responses during growth on glycerol. Microb Cell Factories 11:46
Phue JN, Shiloach J (2005) Impact of dissolved oxygen concentration on acetate accumulation and physiology of E. coli BL21, evaluating transcription levels of key genes at different dissolved oxygen conditions. Metab Eng 7:353–363
San KY, Bennett GN, Berríos-Rivera SJ, Vadali RV, Yang YT, Horton E, Rudolph FB, Sanyar B, Blackwood K (2002) Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab Eng 4:182–192
Schmid JW, Mauch K, Reuss M, Gilles ED, Kremling A (2004) Metabolic design based on a coupled gene expression—metabolic network model of tryptophan production in Escherichia coli. Metab Eng 6:364–377
Shen T, Liu Q, Xie XX, Xu QY, Nl C (2012) Improved production of tryptophan in genetically engineered Escherichia coli with TktA and PpsA overexpression. J Biomed Biotechnol. doi:10.1155/2012/605219
Shi IY, Stansbury J, Kuzminov A (2005) A defect in the acetyl coenzyme A < - > acetate pathway poisons recombinational repair-deficient mutants of Escherichia coli. J Bacteriol 187:1266–1275
Vadali RV, Bennett GN, San KY (2004) Applicability of CoA/acetyl-CoA manipulation system to enhance isoamyl acetate production in Escherichia coli. Metab Eng 6:294–299
Wang J, Cheng LK, Wang J, Liu Q, Shen T, Chen N (2013a) Genetic engineering of Escherichia coli to enhance production of L-tryptophan. Appl Microbiol Biotechnol 97:7587–7596
Wang J, Huang J, Shi JM, Xu QY, Xie XX, Chen N (2013b) Fermentation characterization of an L-tryptophan producing Escherichia coli strain with inactivated phosphotransacetylase. Ann Microbiol 63:1219–1224
Zawada J, Swartz J (2005) Maintaining rapid growth in moderate-density Escherichia coli fermentations. Biotechnol Bioeng 89:407–415
Zhao ZJ, Zou C, Zhu YX, Dai J, Chen S, Wu D, Wu J, Chen J (2011) Development of L-tryptophan production strains by defined genetic modification in Escherichia coli. J Ind Microbiol Biotechnol 38:1921–1929
Zhou L, Tian KM, Zuo ZR, Chen XZ, Shi GY, Singh S, Wang ZX (2011) Elimination of succinate and acetate synthesis in recombinant Escherichia coli for D-lactate production. Chin J Biotechnol 27(1):31–40
Zhu HF, Shimizu K (2005) Effect of a single-gene knockout on the metabolic regulation in Escherichia coli for D-lactate production under microaerobic condition. Metab Eng 7:104–115
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program: 2012AA02A703), and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT 1166).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, C., Cheng, L., Wang, J. et al. Impact of deletion of the genes encoding acetate kinase on production of L-tryptophan by Escherichia coli . Ann Microbiol 66, 261–269 (2016). https://doi.org/10.1007/s13213-015-1103-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-015-1103-4