Abstract
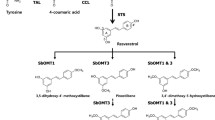
Pterostilbene (3,5-dimethoxy-4′-hydroxyl-trans-stilbene)—a derivative of resveratrol—is a natural dietary compound and the primary antioxidant component in berries. Pterostilbene has significant advantages over resveratrol in bioavailability, half-life in the body, cellular uptake, oral absorption and metabolic stability. Here, we expressed the resveratrol O-methyltransferase (ROMT) gene (VvROMT) from grape (Vitis vinifera) in Escherichia coli and Saccharomyces cerevisiae and confirmed its specific ability to catalyze the production of pterostilbene from resveratrol. By co-expressing an additional two genes from the resveratrol biosynthetic pathway—4-coumarate CoA-ligase (4CL) and stilbene synthase (STS)—a large amount of pterostilbene was produced, with a trace amount of pinostilbene detected. To understand the molecular basis of the catalytic activity, four key amino acid residues were identified in a 3D-model of VvROMT and mutagenized and assayed for augmented catalytic activity. Our results demonstrate the potential utility of the engineered microorganisms for pterostilbene production and provide protein engineering targets that will hopefully lead to increased activity of the ROMT enzyme.





Similar content being viewed by others
References
Adrian M, Jeandet P, Douillet-Breuil AC, Tesson L, Bessis R (2000) Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J Agric Food Chem 48:6103–6105
Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM (2002) Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med 33:387–398
Baerson SR, Dayan FE, Rimando AM, Nanayakkara NP, Liu CJ, Schroder J, Fishbein M, Pan Z, Kagan IA, Pratt LH, Cordonnier-Pratt MM, Duke SO (2008) A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs. J Biol Chem 283:3231–3247
Becker JV, Armstrong GO, van der Merwe MJ, Lambrechts MG, Vivier MA, Pretorius IS (2003) Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res 4:79–85
Beekwilder J, Wolswinkel R, Jonker H, Hall R, de Vos CH, Bovy A (2006) Production of resveratrol in recombinant microorganisms. Appl Environ Microbiol 72:5670–5672
Campagna M, Rivas C (2010) Antiviral activity of resveratrol. Biochem Soc Trans 38:50–53
Chang J, Rimando A, Pallas M, Camins A, Porquet D, Reeves J, Shukitt-Hale B, Smith MA, Joseph JA, Casadesus G (2012) Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol Aging 33:2062–2071
Chemler JA, Koffas MA (2008) Metabolic engineering for plant natural product biosynthesis in microbes. Curr Opin Biotechnol 19:597–605
Chiron H, Drouet A, Lieutier F, Payer HD, Ernst D, Sandermann H Jr (2000) Gene induction of stilbene biosynthesis in Scots pine in response to ozone treatment, wounding, and fungal infection. Plant Physiol 124:865–872
Cunningham FX Jr, Lafond TP, Gantt E (2000) Evidence of a role for LytB in the nonmevalonate pathway of isoprenoid biosynthesis. J Bacteriol 182:5841–5848
Fulda S (2010) Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov Today 15:757–765
Grosdidier A, Zoete V, Michielin O (2011) SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res 39:W270–W277
Guo C, Sinnott B, Niu B, Lowry MB, Fantacone ML, Gombart AF (2013) Synergistic induction of human cathelicidin antimicrobial peptide gene expression by vitamin D and stilbenoids. Mol Nutr Food Res. doi:10.1002/mnfr.201300266
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Jeandet P, Delaunois B, Aziz A, Donnez D, Vasserot Y, Cordelier S, Courot E (2012) Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene, resveratrol. J Biomed Biotechnol 2012:579089
Jeong YJ, An CH, Woo SG, Jeong HJ, Kim YM, Park SJ, Yoon BD, Kim CY (2014) Production of pinostilbene compounds by the expression of resveratrol O-methyltransferase genes in Escherichia coli. Enzym Microb Technol 54:8–14
Joseph JA, Fisher DR, Cheng V, Rimando AM, Shukitt-Hale B (2008) Cellular and behavioral effects of stilbene resveratrol analogues: implications for reducing the deleterious effects of aging. J Agric Food Chem 56:10544–10551
Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL (2011) Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol 68:593–601
Khlebnikov A, Datsenko KA, Skaug T, Wanner BL, Keasling JD (2001) Homogeneous expression of the P(BAD) promoter in Escherichia coli by constitutive expression of the low-affinity high-capacity AraE transporter. Microbiology 147:3241–3247
Krissinel E, Henrick K (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D60:2256–2268
Lavid N, Wang J, Shalit M, Guterman I, Bar E, Beuerle T, Menda N, Shafir S, Zamir D, Adam Z, Vainstein A, Weiss D, Pichersky E, Lewinsohn E (2002) O-methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physiol 129:1899–1907
Lee CM, Su YH, Huynh TT, Lee WH, Chiou JF, Lin YK, Hsiao M, Wu CH, Lin YF, Wu AT, Yeh CT (2013) Blue berry isolate, pterostilbene, functions as a potential anticancer stem cell agent in suppressing irradiation-mediated enrichment of hepatoma stem cells. Evid Based Complement Alternat Med 2013:258425
Lim CG, Fowler ZL, Hueller T, Schaffer S, Koffas MA (2011) High-yield resveratrol production in engineered Escherichia coli. Appl Environ Microbiol 77:3451–3460
Lin HS, Yue BD, Ho PC (2009) Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed Chromatogr 23:1308–1315
Mageroy MH, Tieman DM, Floystad A, Taylor MG, Klee HJ (2012) A Solanum lycopersicum catechol-O-methyltransferase involved in synthesis of the flavor molecule guaiacol. Plant J 69(6):1043–1051
Pari L, Satheesh MA (2006) Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats. Life Sci 79:641–645
Park ES, Lim Y, Hong JT, Yoo HS, Lee CK, Pyo MY, Yun YP (2010) Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway. Vasc Pharmacol 53:61–67
Paul B, Masih I, Deopujari J, Charpentier C (1999) Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. J Ethnopharmacol 68:71–76
Paul S, Rimando AM, Lee HJ, Ji Y, Reddy BS, Suh N (2009) Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev Res (Phila) 2:650–657
Remsberg CM, Yanez JA, Ohgami Y, Vega-Villa KR, Rimando AM, Davies NM (2008) Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother Res 22:169–179
Riche DM, McEwen CL, Riche KD, Sherman JJ, Wofford MR, Deschamp D, Griswold M (2013) Analysis of safety from a human clinical trial with pterostilbene. J Toxicol 2013:463595
Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO (2002) Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem 50:3453–3457
Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR (2004) Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J Agric Food Chem 52:4713–4719
Rimando AM, Nagmani R, Feller DR, Yokoyama W (2005) Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem 53:3403–3407
Rimando AM, Pan Z, Polashock JJ, Dayan FE, Mizuno CS, Snook ME, Liu CJ, Baerson SR (2012) In planta production of the highly potent resveratrol analogue pterostilbene via stilbene synthase and O-methyltransferase co-expression. Plant Biotechnol J 10:269–283
Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738
Schmidlin L, Poutaraud A, Claudel P, Mestre P, Prado E, Santos-Rosa M, Wiedemann-Merdinoglu S, Karst F, Merdinoglu D, Hugueney P (2008) A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol 148:1630–1639
Sydor T, Schaffer S, Boles E (2010) Considerable increase in resveratrol production by recombinant industrial yeast strains with use of rich medium. Appl Environ Microbiol 76:3361–3363
Trantas E, Panopoulos N, Ververidis F (2009) Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab Eng 11:355–366
Tu Y, Rochfort S, Liu Z, Ran Y, Griffith M, Badenhorst P, Louie GV, Bowman ME, Smith KF, Noel JP, Mouradov A, Spangenberg G (2010) Functional analyses of caffeic acid O-Methyltransferase and Cinnamoyl-CoA-reductase genes from perennial ryegrass (Lolium perenne). Plant Cell 22:3357–3373
Urban P, Mignotte C, Kazmaier M, Delorme F, Pompon D (1997) Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J Biol Chem 272:19176–19186
Wang Y, Yu O (2012) Synthetic scaffolds increased resveratrol biosynthesis in engineered yeast cells. J Biotechnol 157:258–260
Wang Y, Chen H, Yu O (2010) Metabolic engineering of resveratrol and other longevity boosting compounds. Biofactors 36:394–400
Wang Y, Halls C, Zhang J, Matsuno M, Zhang Y, Yu O (2011) Stepwise increase of resveratrol biosynthesis in yeast Saccharomyces cerevisiae by metabolic engineering. Metab Eng 13:455–463
Watts KT, Lee PC, Schmidt-Dannert C (2006) Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol 6:22
Weickert MJ, Doherty DH, Best EA, Olins PO (1996) Optimization of heterologous protein production in Escherichia coli. Curr Opin Biotechnol 7:494–499
Wen X, Walle T (2006) Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos 34:1786–1792
Wilson MA, Rimando AM, Wolkow CA (2008) Methoxylation enhances stilbene bioactivity in Caenorhabditis elegans. BMC Pharmacol 8:15
Wu J, Liu P, Fan Y, Bao H, Du G, Zhou J, Chen J (2013) Multivariate modular metabolic engineering of Escherichia coli to produce resveratrol from L-tyrosine. J Biotechnol 167:404–411
Zhang Y, Li SZ, Li J, Pan X, Cahoon RE, Jaworski JG, Wang X, Jez JM, Chen F, Yu O (2006) Using unnatural protein fusions to engineer resveratrol biosynthesis in yeast and Mammalian cells. J Am Chem Soc 128:13030–13031
Zubieta C, He XZ, Dixon RA, Noel JP (2001) Structures of two natural product methyltransferases reveal the basis for substrate specificity in plant O-methyltransferases. Nat Struct Biol 8:271–279
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program, 2013AA102801-03).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2510 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Bhuiya, M.W., Zhou, R. et al. Pterostilbene production by microorganisms expressing resveratrol O-methyltransferase. Ann Microbiol 65, 817–826 (2015). https://doi.org/10.1007/s13213-014-0922-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-014-0922-z