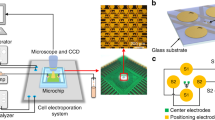
Abstract
This paper presents a cell electroporation characterization chip using a single tapered channel for a continuous gradient of electric fields. In the cell electroporation study, it is required to find the optimal electric field strength for obtaining the maximum number of both viable and electroporated cells possible. The previous electroporation chip with a single stepwise channel generated a limited number of electric fields. However, the present chip, where continuous electric fields (0.3–.5 kV/cm) are generated by a pair of external electrodes across a tapered single channel, provides a more finely tuned electric field for stable cell electroporation with high viability. In the experimental study, we characterize H23 non-small-cell lung cancer (NSCLC) cells. In the previous electroporation chip, the percentages of viable electroporated H23 cells were 51.4% and 44.5% at 0.4 and 0.45 kV/cm, respectively. The present chip was measured to have an electric field of 0.44 kV/cm with a maximum percentage of viable electroporated cells of 54.7±4.8%. The present cell electroporation characterization chip has potential for use in integrated cell chips to find fine optimal electric field conditions for the study of electroporation.
Similar content being viewed by others
References
Weaver, J.C. Electroporation of Biological Membranes from Multicellular to Nano Scales. IEEE Trans. Dielectr. Electr. Insul. 10, 754–68 (2003).
Prasanna, G.L. & Panda, T. Electroporation: basic principles, practical considerations and applications in molecular biology. Bioprocess. Eng. 16, 261–64 (1997).
Friedrich, U. et al. High efficiency electrotransfection with aluminum electrodes using microsecond controlled pulses. Bioelectrochem. Bioenerg. 47, 103–11 (1998).
Kim, J.A. et al. A multi-channel electroporation microchip for gene transfection in mammalian cells. Biosens. Bioelectron. 22, 3273–277 (2007).
Jain, T. & Muthuswamy, J. Bio-chip for spatially controlled transfection of nucleic acid payloads into cells in a culture. Lab Chip 7, 1004–011 (2007).
Valero, A. et al. Gene transfer and protein dynamics in stem cells using single cell electroporation in a microfluidic device. Lab Chip 8, 62–7 (2008).
Santra, T.S. & Tseng, F.G. Recent trends on Micro/Nanofluidic single cell electroporation. Micromachines 4, 333–56 (2013).
Geng, T. & Lu, C. Microfluidic electroporation for cellular analysis and delivery. Lab Chip 13, 3803–821 (2013).
Baron, S., Poast, J., Rizzo, D., Mcfarland, E. & Kieft, E. Electroporation of antibodies, DNA, and other macromolecules into cells: a highly efficient method. J. Immunol. Methods 242, 115–26 (2000).
Ho, S.Y., Mittal, G.S. & Cross, J.D. Effects of High Field Electric Pulses on the Activity of Selected Enzymes. J. Food Eng. 31, 69–4 (1997).
Kang, W. et al. Nanofountain probe electroporation (NFP-E) of single cells. Nano Lett. 13, 2448–457 (2013).
Serpersu, E.H. & Tsong, T.Y. Activation of Electrogenic Rb+ Transport of (Na,K)-ATPase by an Electric Field. J. Biol. Chem. 259, 7155–162 (1984).
Serpersu, E.H., Kinosita, K. Jr., & Tsong, T.Y. Reversible and irreversible modification of erythrocyte membrane permeability by electric field. Biochim. Biophys. Acta. 812, 779–85 (1985).
Choi, Y.S., Kim, H.-B., Kwon, G.-S. & Park, J.-K. Onchip testing device for electrochemotherapeutic effects on human breast cells. Biomed. Microdevices 11, 151–59 (2009).
Boukany, P.E. et al. Nanochannel electroporation delivers precise amounts of biomolecules into living cells. Nat. Nanotechnol. 6, 747–54 (2011).
Santra, T.S., Wang, P.-C., Chang, H.-Y. & Tseng, F.-G. Tuning nano electric field to affect restrictive membrane area on localized single cell nano-electroporation. Appl. Phys. Lett. 103, 233701 (2013).
Xie, X. et al. Nanostraw-electroporation system for highly efficient intracellular delivery and transfection. ACS Nano 7, 4351–358 (2013).
Bao, N., Le, T.T., Cheng, J.-X. & Lu, C. Microfluidic electroporation of tumor and blood cells: observation of nucleus expansion and implications on selective analysis and purging of circulating tumor cells. Integr. Biol. (Camb). 2, 113–20 (2010).
Lin, Y.-C., Jen, C.-M., Huang, M.-Y., Wu, C.-Y. & Lin, X.-Z. Electroporation microchips for continuous gene transfection. Sens. Actuators, B 79, 137–43 (2001).
Shin, Y.S. et al. Electrotransfection of Mammalian Cells Using Microchannel-Type Electroporation Chip. Anal. Chem. 76, 7045–052 (2004).
Fox, M.B. et al. Electroporation of cells in microfluidic devices: a review. Anal. Bioanal. Chem. 385, 474–85 (2006).
Kim, M.-J., Kim, T. & Cho, Y.-H. Cell electroporation chip using multiple electric field zones in a single channel. Appl. Phys. Lett. 101, 223705 (2012).
Lee S.-W. & Tai, Y.-C. A micro cell lysis device. Sens. Actuators, A 73, 74–9 (1999).
Khine, M., Lau, A., Zanetti, C.I., Seo, J. & Lee, L.P. A single cell electroporation chip. Lab Chip 5, 38–3 (2005).
Lee, D.W. & Cho, Y.-H. A continuous electrical cell lysis device using a low dc voltage for a cell transport and rupture. Sens. Actuators, B 124, 74–9 (2007).
Gabriel, B. & Teissié, J. Control by electrical parameters of short- and long-term cell death resulting from electropermeabilization of Chinese hamster ovary cells. Biochim. Biophys. Acta. 1266, 171–78 (1995).
Rubinsky, B. Irreversible Electroporation in Medicine. Technol. Cancer Res. Treat. 6, 255–59 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, MJ., Kim, T., Doh, I. et al. A cell electroporation characterization chip using a single tapered channel for continuous electric field variation. BioChip J 8, 269–274 (2014). https://doi.org/10.1007/s13206-014-8404-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-014-8404-8