Abstract
In the present study, we demonstrate magnetic iron (III) oxide nanoparticles (Fe2O3 NPs) uptake by the Solanum lycopersicum (S. lycopersicum) plant. The S. lycopersicum seeds were coated with Fe2O3 NPs and allowed to germinate in moistened sand bed. The seedlings are observed for 20 days, and then, it was post-treated using different amounts of Fe2O3 NPs in hydroponic solution for 10 days. The plant was allowed to grow in green house for 3 months, and uptake of NPs through roots and translocation into different parts was studied. For this, we have segmented the plants and incubated with 10 % NaOH solution. It is found that the NPs are deposited preferentially in root hairs, root tips followed by nodal and middle zone of plant. The iron present in the whole plant was quantitatively estimated by treating dry biomass of the plant in acid. The Fe2+/Fetotal increased with increasing concentration of NPs and >45 % ferrous iron suggests the biomineralization of NPs due to rich phytochemicals in plants. We believe that the present study is useful to build a base line data for novel applications in agri-nanotechnology.
Similar content being viewed by others
Introduction
Nanotechnology has great potential as it can enhance the quality of human life through its applications in diverse field such as agriculture and food technology (Buzea et al. 2007; Walker and Bucher 2009). However, as with any new technology, unforeseen risks might cause potential hazards. Nevertheless, great potential of nanoscience and technology lies in the provision of state-of-the-art solutions for various challenges faced in agriculture and future society. Certain hot issues such as climate change, depletion of natural resources and agriculture productivity may be tackled with the use of nanotechnology. The development of nanodevices and nanomaterials has emerged as promising tools for the conversion of agricultural and bio-wastes into energy, increasing agricultural by-products through enzymatic nano-bio-processing, disease control, treatment of plants using nanocides, etc. (Nair et al. 2010). The fertilizers are very important in plants growth, but most of the applied fertilizers are frequently lost due to the degradation by photolysis, leaching, hydrolysis, decomposition, etc. Thus, it is more essential to reduce nutrient losses in fertilization and to increase the crop yield through the advancement of new nanotechnology applications. Nanomaterials-based fertilizers and/or nanomaterials functionalized nutrients might have the properties such as crops improvement, specifically targeted, slow release of nutrients that regulate plant growth, less eco-toxicity, remotely regulated and other multifunctional characteristics to avoid biological blockade for successful targeting (Vasir and Labhasetwar 2007; Agasti et al. 2010; Nair et al. 2010). Plants are an important component in the ecological system, which provides a potential pathway for NPs transport to the environment, and serve as a significant route for their bioaccumulation into the food chain. The rates of NPs uptake, intracellular localization as well as biomineralization in different plants have been reported (Mailander and Landfester 2009; Verma and Stellacci 2010; Delehanty et al. 2009). These studies reveal that it is difficult to generalize the nature of NPs for their improved efficiency or productivity in plants. Furthermore, the rate and mechanism of uptake turns out to be cell-type-dependent and morphological features of NPs. The sieving properties are determined by pore diameter of cell wall ranging from 5 to 20 nm (Fleischer et al. 1999). Hence, the NPs with a diameter <20 nm could be easily pass through and reach the plasma membrane (Chithrani and Chan 2007; Jin et al. 2009). The recent developments in plant science and agriculture cover the application of NPs for more effective and safe use of chemicals (ca: agrochemicals) for plants, plants as source for metal/metal oxide NPs synthesis, the effects of different NPs on growth as well as metabolic functions of different plants and NPs-mediated plant genetic transformation.
There are few reports on the effects of NPs in plants, which have focused mainly on phytotoxicity and how certain plant metabolic functions are affected. These results vary with nature of NPs and the plant species, and are not always consistent between different studies. For instance, root elongation was enhanced in cucumber and onion plant when carbon nanotubes (CNTs) have been applied, while such CNTs reduced root elongation in letttce and tomato plant (Canas et al. 2008). In another study, titania (TiO2) NPs were shown to enhance the growth of spinach plant (Zheng et al. 2005), whereas alumina (Al2O3) NPs were reported to inhibit root elongation of different plants such as soybean, corn, cucumber, cabbage and carrot (Yang and Watts 2005). In earlier study, (Lin and Xing 2007) have documented the effects of different types of NPs such as multi-walled carbon nanotube (MWCNTs), alumina, aluminum, zinc oxide and zinc on seed germination and root elongation of six plant species, namely radish, ryegrass, grape, lettuce, cucumber and corn, respectively, which showed that seed germination was not affected in most of the cases, while root elongation was inhibited. Due to their different morphologies of nanomaterials, it is difficult to predict the positive or negative effect in their mode of action in environment and within living systems (Holsapple et al. 2005). In fact, the phytotoxic affects of MWCNTs (rice) (Lin et al. 2009), magnetic NPs (pumpkin) (González-Melendi et al. 2008; Zhu et al. 2008), copper NPs (zucchini) (Stampoulis et al. 2009) and silver NPs (onion, pearl millet) (Kumari et al. 2009; Parveen and Rao 2015) have been studied in addition to their effect on seed germination and elongation of root/plant growth. These studies show contradictory results (both positive and negative effect) on different plant species when the same nanomaterial is used.
Among the above NPs, iron oxide-based NPs are more advantages as they find specific localization to release their load, which is of great interest in the study of nanoparticulate delivery in plants. In addition, iron oxide NPs are environmentally benign, readily available or easy to synthesize, magnetically sensitive, redox active and bio-functionalizable. Iron is an important microelement related to many physiological reactions and is an important component of chlorophyll. Moreover, the phytotoxicity profile of NPs has also been investigated by researchers via seed germination and root elongation tests, which showed no toxicity effects of NPs on plant physiologies (González-Melendi et al. 2008; Zhu et al. 2008). Thus, Fe-oxides are relatively safe for nanoparticulate delivery in plants. Hence, an attempt was made here to determine the effect of Fe2O3 NPs on seed germination and plant growth of S. lycopersicum plant. The significant uptake of Fe2O3 by S. lycopersicum plant including their subsequent translocation and accumulation in different tissue was also demonstrated here. The biomineralization aspect is also examined based on the reduction in ferric to ferrous iron.
Experimental details
Materials
Solanum lycopersicum was selected as a model plant because of its large water uptake capacity and was grown hydroponically in a growth medium. S. lycopersicum seeds were used for determining the effect of magnetic Fe2O3 NPs on seed germination, seedling vigor and plant growth in the green house. S. lycopersicum seeds were collected from commercial market at Tarikere, Chikmagalur district of Karnataka, India. The spherical-shaped magnetic Fe2O3 NPs with an average particle size of below 10 nm selected for this study was obtained from Vergent System Pvt. Ltd., Bangalore, India. The oxide was brown red in color probably due to partial oxidation to α-Fe2O3 (red oxide). A stock solution containing 10 g of Fe2O3 NPs in 200 mL of distilled water is prepared, and from this, 50, 100, 200, 400 and 800 mgL−1 of solution were obtained. The optimum solutions were stored in glass bottles separately at room temperature for further experiments.
Seed germination
Solanum lycopersicum seeds were cleaned with liquid soap solution and distilled water to remove the dust and other surface adherents. The cleaned and dried seeds (400 seeds) were taken in a test tube and treated with 1 mL of distilled water (as control) and 50, 100, 200, 400 and 800 mgL−1 of prepared Fe2O3 NPs solution. This was shaken gently and incubated for 12 h at 50 °C. The treated and untreated seeds were incubated by the standard blotter method, as per the rules of International Seed Testing Association (ISTA, 2003). Briefly, all seeds were subsequently transferred into moistened blotter disks containing three pieces of blotter paper. A 10–25 seeds were placed on wet blotting paper kept at the bottom of Petri dish maintaining equal distance among the seeds. Thereafter, 1 mL suspensions of 50, 100, 200, 400 and 800 mgL−1 of Fe2O3 NPs were added. Finally, the disks were covered and allowed for seed germination in incubator at 20 ± 2 °C for 7 days. The experiment was performed in triplicate. The percentage of seed germination was calculated as:
Seedling vigor
The cleaned and dried seeds were taken in a test tube and treated with 1 mL of distilled water (as control) and solution of Fe2O3 NPs (concentration as mentioned in section above). This was shaken gently and incubated for 12 h at 50 °C. Then, the treated and untreated seeds were placed on moistened paper towel prepared as three-layered roll. The prepared rolls were kept in a beaker containing 3 mL distilled water and subsequently placed in incubation at 20 ± 2 °C for 10 days, ISTA 2003. Then, they were separated and the root and shoot lengths were measured by using digimatic calipers (Mitutoyo Rochester, New York). The experiment was performed in triplicate, and the vigor index was calculated as:
Fe2O3 NPs on seedling growth in green house
Solanum lycopersicum seeds were soaked on autoclaved (121 °C, 15 lb pounds, for 20 min on three consecutive days) sand bed with moistened 38 × 27 cm plastic trays. Then, the seeds were allowed to germinate at room temperature for 20 days. The sand bed was irrigated daily using 10 % nutrients solution. After 20 days, the seedlings were removed carefully from the sand bed with intact roots and washed with slow running tap water to dislodge sand particles adhered to the seedling roots. They were placed in plastic cups (20 × 10 cm) containing 1 mL of 50, 100, 200, 400 and 800 mgL−1 Fe2O3 NPs solution with 5 mL nutrients solution. The NPs solution in cup was shaken gently at frequent intervals for the purpose of NPs to lodge on the seedling root surface. After 2 days, 10 mL of fresh nutrient solution was added and incubation was continued for 7 days. Seedlings that were treated and untreated with NPs were transplanted to a pot (30 × 8 cm) containing the blotting mixture (red soil: sand = 3:1). The potted seedlings were grown in the greenhouse which received the natural solar illumination. These were brought to the laboratory, and roots were washed with tap water to remove the soil. The root and shoot lengths were observed at the end of 3, 6 and 9 weeks after the treatment of NPs and measured vigor index. The seedlings intact with roots were blotted to remove excess of moisture, and the fresh biomass was determined. The same seedlings were dried in hot air oven at 60 °C to obtain dry biomass (g).
Movement and localization of NPs
The 3-week cultured seedlings (NPs treated) were removed from the soil, and roots were washed with water. Seedlings were subjected to maceration by immersing them in 10 % NaOH for 48 h and washed with distilled water to remove the excess NaOH. The segmented root, stem and leaves (1 cm length) were placed on a glass slide and covered with a cover slip and gently tapped for uniform spread of the tissue to observe under compound microscope (10×). The NPs localized in different segments of seedling were determined by observing the distinct brownish red color crystals in plant tissues.
Iron present in the dry biomass of plants (during seedling growth)
One gram each of the dry biomass of the plants (which were exposed to different amounts of Fe2O3 NPs, 0–800 mgL−1, during seedling growth) was subjected to iron extraction by treating with 1 mL HCl for about 24 h. This solution was analyzed for ferrous and ferric iron by 1,10-phenanthroline method using UV–Vis spectrophotometer as follows.
One milliliter of the above iron sample was added to 50-mL volumetric flask containing 1 mL of dil. HCl. Then, 5 mL of 10 % hydroxylamine hydrochloride (reducing agent, which reduces all the ferric to ferrous iron, Fe3+ → Fe2+), was added and allowed to stand for 5 min. To this mixture, 2.5 mL of buffer solution (NH3/NH4OH) and 1 mL of 50 % ammonia were added. Finally, 5.0 mL of 0.25 % of 1,10-phenanthroline solution was added and diluted up to the mark with distilled water. The absorption of orange-red color thus developed was measured at 510 nm. Based on the calibration plot obtained, the concentration was estimated using the formula:
To know the concentration of ferrous iron (Fe2+), the above procedure is followed without adding a reducing agent, hydroxylamine hydrochloride. The amount of ferric iron, Fe3+ = (Fetotal − Fe2+).
Results and discussion
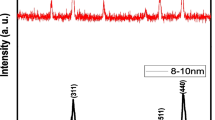
The effect of magnetic Fe2O3 NPs on seed germination, seedling growth and NPs uptake in S. lycopersicum plant is studied in the present work. Figure 1 shows transmission electron microscopic (TEM) image and X-ray diffraction (XRD) pattern of the Fe2O3 NPs used here. It is clear from TEM images that the particle sizes of Fe2O3 NPs are <10 nm and are well dispersed. The XRD pattern confirmed the cubic crystal structure of γ-Fe2O3 (JCPDS card No. 39-1346, maghemite). The oxide was brown red in color probably due to partial oxidation to hematite (α-Fe2O3, red oxide). Both maghemite (ccp) and hematite (hcp) contain Fe3+ ions in their lattice.
The locations of Fe2O3 NPs in different parts of 3-week-old S. lycopersicum plant were observed in compound microscopic images of the sliced parts (not shown here). The NPs uptake through root tissue and its deposition or translocation throughout the plant is observed clearly. A brownish red colored Fe2O3 particles seemed to aggregate in the intracellular regions. It is found that the NPs are deposited preferentially in root hair, root tip followed by nodal and middle zone of plant. Also, we have observed the aggregation of NPs in the inner regions of the pith and parenchymatic cells. The NPs can directly enter the plant through the root cells and are gradually demineralized. The Fe2O3 NPs used here are ferromagnetic in nature and exhibit spontaneous magnetization. Thus, the excess (before demineralization) Fe2O3 NPs can have magnetic interaction leading to accumulation or agglomeration of the same at different parts of the plants such as nodal zone, middle zone of root, root hairs and root tips. The in situ analysis could not be studied here. However, Fe content in the dry mass of the same plant showed the enhanced Fe when compared to control. As shown in Table 3, Fetoatal (=ferrous + ferric) present in the dry mass increased with increase in amount of Fe2O3 NPs fed to the plant.
Figure 2 shows the SEM images and their corresponding EDX of 3-week-old S. lycopersicum plants fed with different concentrations of Fe2O3 NPs. In corroboration with compound microscopic images, it is clear from EDX analysis that the Fe is very much present and is increased with the amount of Fe2O3 NPs fed by hydroponic system. Thus, the uptake of Fe2O3 NPs by S. lycopersicum plant is clarified here.
As shown in Table 1, Fe2O3 NPs have not affected the seed living process and plant growth. In fact, Fe2O3 NPs have enhanced the seed germination and plant growth when compared to the control. During germination, the NPs are presumed to diffuse through ‘nano holes’ on seed coats, resulting in improved germination condition, followed by slow and minimal release of NPs. The highest percentage of seed germination was observed to be 93 % at 50 mgL−1 and 97 % at 200 mgL−1. However, when treated at higher concentration (400−800 mgL−1) of NPs, seed germination was slightly reduced (nearly equal to the growing rate of control) probably due to toxicity level of NPs in higher concentration. It indicates that Fe2O3 NPs in the range of 50−200 mgL−1 could be used in agricultural practices. A similar observation was made in the case of TiO2 and Fe2O3 NPs by treating spinach and pumpkin plant, respectively (Gao et al. 2008; Zhu et al. 2008). The biocompatible magnetic fluid NPs have also shown to induce good germination in maize (Racuciu et al. 2009).
Tables 1 also show the root and shoot lengths of S. lycopersicum seedlings on treating with different concentrations of Fe2O3 NPs. The root and shoot lengths were found to increase gradually with an increase in NPs concentration from 50 to 200 mgL−1 and decrease gradually at higher concentration (>200 mgL−1) of NPs treated. The seedling vigor was high at 50 mgL−1 when compared to that of control and decreased gradually with increase in concentration, but it was still higher than that of control. Recently, Parveen and Rao (2015) reported that the Ag NPs caused enhanced seedling growth in Pennisetum glaucum plant when the same amount of NPs was treated. This result was comparable to our study obtained here for Fe2O3 NPs on S. lycopersicum. The beneficial effect of Ag NPs on plants is well documented (Gade et al. 2010; Gardea-Torresdey et al. 2002; Sharma et al. 2007; Gardea-Torresdey et al. 2003; Harris and Bali 2008). The enhanced seedling growth here could be due to change in physiological process. Since the vigor index is based on seedling length and seed germination, the result here shows the overall seed development on treating with Fe2O3 NPs.
The improved seedling growth with respect to shoot and root lengths is evident with the treatment of NPs up to 3 weeks as shown in Table 2. Significant increase in shoot and root lengths was observed here from 50 to 400 mgL−1 of NPs. After 6 weeks, we can see the increased shoot and root lengths even at higher concentration, 800 mgL−1. The vigor index showed significant increase with increasing Fe2O3 NPs. As can be seen in Table 3, the seedling biomass varied with concentration of Fe2O3 NPs treated and growth period. The fresh biomass of seedling increased significantly on treating with 50 mgL−1 of NPs. On the other hand, the dry biomass increased continuously. To our knowledge, there is no information on the effect of Fe2O3 NPs on seedling growth in the greenhouse. However, an attempt has been made to determine the effect of Ag NPs and CNTs on seedling growth, plant growth and yield in hydroponic system (Stampoulis et al. 2009; Kumari et al. 2009).
In order to understand the internalization and/or biomineralization of Fe2O3 NPs present in S. lycopersicum plant here, we have estimated the ferrous and ferric iron from the dry biomass of the plant. For this, 3-week-old S. lycopersicum plants fed with different amounts of Fe2O3 NPs were used. As shown in Table 4, the amount of Fetotal (=Fe2+ + Fe3+) expressed in terms of Fe2O3 is 169, 190, 284, 300, 316 and 390 µg per gram of dry biomass in plants fed with 0, 50, 100, 200, 400 and 800 mgL−1 of Fe2O3 NPs, respectively. The iron present in control experiment (169 µg per gram of dry biomass) is attributed to many physiological reactions (as iron is an important microelement) and is an important component of chlorophyll. Although Fe2O3 contains pure ferric iron, it is very interesting to find that part of this is converted to ferrous iron. The Fe2+/Fetotal ratio increased with the amount of Fe2O3 NPs fed to plants, except at high concentration (800 mgL−1). This could be due to biomineralization of Fe2O3 localized at different parts of S. lycopersicum plant. Such reduction in Fe3+ to Fe2+ is understandable here due to the presence of rich phytochemicals (ca. bioreductants). The Fe2+/Fetotal ratio might vary with the aging of plant and/or residual time of Fe2O3 localization.
Conclusion
In this study, uptake of environmentally benign and biocompatible Fe2O3 NPs by S. lycopersicum plant is tested, and their impact on plant growth is also assessed. The localization of Fe2O3 NPs in the plant body as well as the biomineralization aspect is also examined. The Fe2O3 NPs-coated seeds have shown enhanced seed germination, the root and shoot lengths of S. lycopersicum. Significant amount of Fe2O3 NPs suspended in a liquid medium is taken up by S. lycopersicum plant and translocated throughout the plant tissue. The tested NPs seem to deposit preferentially in root hairs, root tip followed by nodal and middle zone of plant. Based on the increased Fe2+/Fetotal ratio found from the iron extracted from dry biomass of the plant, we confirm the uptake of Fe2O3 NPs and their internalization and/or biomineralization in the plant body. The reduction in ferric to ferrous iron is attributed to the rich phytochemicals in the plant. Overall, this study shows enhanced growth parameters of S. lycopersicum plant by using Fe2O3 NPs and there is no adverse effect (ca. toxicity). These observations encourage the use of NPs for the advantage of agricultural crops. Nevertheless, studies at the molecular level are needed for further assessment and use of Fe2O3 NPs for higher productivity and ‘rest’ resistance, etc.
References
Agasti SS, Rana S, Park MH, Kim CK, You CC, Rotello VM (2010) Nanoparticles for detection and diagnosis. Adv Drug Deliver Rev 62:316–328
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2:17–71
Canas JE, Long M, Nations S, Vadan R, Dai L, Luo M, Ambikapathi R, Lee EH, Olszyk D (2008) Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ Toxicol Chem 27:1922–1931
Chithrani BD, Chan WC (2007) Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett 7:1542–1550
Delehanty JB, Mattoussi H, Medintz IL (2009) Delivering quantum dots into cells: strategies, progress and remaining issues. Anal Bioanal Chem 393:1091–1105
Fleischer A, ONeill MA, Ehwald R (1999) The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol 121:829–838
Gade A, Ingle A, Whiteley C, Rai M (2010) Mycogenic metal nanoparticles: progress and applications. Biotech Lett 32:593–600
Gao F, Hong F, Liu C, Zheng L, Su M, Wu X, Yang F, Wu C, Yang P (2008) Mechanism of nano anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol Trace Elem Res 111:239–253
Gardea-Torresdey JL, Parsons JG, Gomez E, Peralta-Videa J, Troiani HE, Santiago P, Yacaman MJ (2002) Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett 2:397–401
Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Miguel JY (2003) Alfalfa Sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir 19:1357–1361
Gonzalez-Melendi P, Fernández-Pacheco R, Coronado MJ, Corredor E, Testillano PS, Risueño MC, Marquina C, Ibarra MR, Rubiales D, Pérez-de-Luque A (2008) Nanoparticles as smart treatment-delivery systems in plants: assessment of different techniques of microscopy for their visualization in plant tissues. Ann Bot 101:187–195
Harris AT, Bali R (2008) On the formation and extent of uptake of silver nanoparticles by live plants. J Nanopart Res 10:691–695
Holsapple M, Farland W, Landry T, Monteiro-Riviere N, Carter J, Walker N, Thomas K (2005) Research strategies for safety evaluation of nanomaterials, Part II: toxicological and safety evaluation of nanomaterials, current challenges and data needs. Toxicol Sci 88:12–17
Jin H, Heller DA, Sharma R, Strano MS (2009) Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS Nano 3:149–158
Vasir Jk, Labhasetwar V (2007) Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv Drug Deliver Rev 59:718–728
Kumari M, Mukherjee A, Chandrasekaran N (2009) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407:5243–5246
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Lin S, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova TA, Rao AM, Luo H, Ke PC (2009) Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 5:1128–1132
Mailander V, Landfester K (2009) Interaction of nanoparticles with cells. Biomacromolecules 10:2379–2400
Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163
Parveen A, Rao S (2015) Effect of nanosilver on seed germination and seedling growth in pennisetum glaucum. J Cluster Sci 26:693–701
Racuciu M, Miclaus S, Creanga DE (2009) The response of plant tissues to magnetic fluid and electromagnetic exposure. Rom J Biophys 19:73–83
Sharma NC, Sahi SV, Nath S, Parsons JG, Gardea-Torresdey JL, Pal T (2007) Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ Sci Tech 41:5137–5142
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Tech 43:9473–9479
Verma A, Stellacci F (2010) Effect of surface properties on nanoparticle-cell interactions. Small 6:12–21
Walker NJ, Bucher JR (2009) A 21st century paradigm for evaluating the health hazardous of nanoscale materials? Toxicol Sci 110:251–254
Yang L, Watts DJ (2005) Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett 158:122–132
Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO(2) on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–92
Zhu H, Han J, Xiao JQ, Jin Y (2008) Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit 10:713–717
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Shankramma and S. Yallappa have contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shankramma, K., Yallappa, S., Shivanna, M.B. et al. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl Nanosci 6, 983–990 (2016). https://doi.org/10.1007/s13204-015-0510-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-015-0510-y