Abstract
An analytical method using modified SiO2 nanoparticles as solid-phase extractant has been developed for the preconcentration of trace amounts of Hg(II) in different water samples. Conditions of the analysis such as preconcentration factor, effect of pH, sample volume, shaking time, elution conditions and effects of interfering ions for the recovery of analyte were investigated. The adsorption capacity of nanometer SiO2-APTMS was found to be 181.42 µmol g−1 at optimum pH and the detection limit (3σ) was 0.45 µg L−1. The extractant showed rapid kinetic sorption. The adsorption equilibrium of Hg(II) on nanometer SiO2-APTMS was achieved just in 15 min. Adsorbed Hg(II) was easily eluted with 4 mL of 2.0 M hydrochloric acid. The maximum preconcentration factor was 75. The method was applied for the determination of trace amounts of Hg(II) in various synthetic samples and water samples.
Similar content being viewed by others
Introduction
In recent years, the toxicity and effects of trace elements to the danger of public health and environment have attracted increasing attention in the fields of pollution and nutrition. Hg(II) and its organometallic species are considered to be highly toxic to the environment, especially the aquatic system. Hg(II) is leached from rocks and soils into water system by natural processes, some of which are accelerated by human activities. The World Health organization (WHO) recommends a limit of l µg L−1 of Hg(II) in drinking water. Direct determination of mercury element at trace levels by conventional method is limited on account of its low concentration and matrix interferences. In trace analysis, therefore preconcentration or separation technique is frequently necessary to improve the detection limit and selectivity. It has become necessary to develop an accurate and sensitive method for quantitative determination of Hg(II) the environmental samples. One of the most important objectives for analytical chemists is metal quantification at low concentration level below µg L−1 or ng mL−1. Despite recent progress in instrumental techniques and increases in the selectivity and sensitivity of analytical techniques, direct determination of trace elements in sample is still difficult. Therefore, preconcentration and selective separation of trace elements from different water samples is very important and need much more attention.
Therefore, accurate and reliable methods sometimes must be developed for mercury determination. Because of their extremely low concentration, a preliminary concentration step i.e., preconcentration is usually necessary prior to the determination of the metals (Yaman 1999; Anezaki et al. 1998; Zolotov et al. 1995; Karadjova 1995; Shar and Soomaro 2004; Qufen et al. 2002). Nowadays, many separation/preconcentration techniques for metal ions determination include liquid–liquid extraction, (Liska 1993; Martinez et al. 2000; Bruzzoniti et al. 2000) ion-exchange resins, (Cao and Fang 1999; Yang et al. 2004) resin chelation and solid-phase extraction (Alonso et al. 2001; Henglein 1989; Panigrahi et al. 2004). Of all theses methods, solid phase extraction has been widely used in comparison with traditional extraction techniques; since it is simple, rapid and inexpensive, less polluting to the environment and can be easily automated. Nowadays, nanometer materials have become more important owing to its special physical and chemical properties. The field of nanocomposite materials has received the attention, imagination and close scrutiny of scientists and engineer in recent years. These particles fall within the colloidal range, exhibiting typical colloidal properties. One of the specific properties of nanomaterials is that a high percent of atoms of the nanoparticle is on the surface. The surface atoms are unsaturated and can therefore bind with other atoms, possess high chemical activity. Nanoparticles exhibit intrinsic surface reactivity and high surface areas and can strongly chemisorb many substances. The size, surface structure and interparticle interaction of nanomaterials determine their unique properties and the improved performances and make their potential application in many areas (Panigrahi et al. 2004; Okuyama and Lenggoro 2003). Nanoparticles such as TiO2 (Schmidt 2001; Hosono et al. 2004), Al2O3 (Hiraide et al. 1997), ZrO2 (Sarkar et al. 2003), CeO2 (Ragai and Selim 1987) and modified silica nanoparticles have been used for the preconcentration of many metal ions and give promising results when used for trace element analysis of different samples. In present work, chemically grafted SiO2-APTMS nanoparticles were prepared by sol–gel method and characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR). These nanoparticles have been used for the preconcentration and separation of cadmium prior to their determination by spectrophotometric method.
Experimental
Apparatus
Absorbance of Hg(II) was measured with UV–Vis Shimadzu-1700 spectrophotometer. The pH values were controlled by century Cp-901 digital pH meter. Infrared spectra were recorded on a Perkin Elmer FT-IR analysis.
Reagents and standard solutions
All reagents used were of analytical grade unless otherwise stated and all solutions were prepared in double distilled deionized water. 3-Aminopropyltrimethoxysilane (APTMS) of GR grade was supplied by Acros Organics (Mumbai). The glassware was washed with chromic acid and soaked in 5 % nitric acid overnight and then cleaned with double distilled water before use. The pH adjustments were made with hydrochloric acid and sodium hydroxide and buffer solutions of acetic acid and sodium acetate, ammonia and ammonium chloride, boric acid and sodium tetraborate were prepared to maintain the pH of the solution.
Modification process
Surface modification of SiO2 nanoparticles were performed in a 250 mL flask. Nanometer SiO2 (1 g) was dispersed into dry toluene (30 mL), and then 3-aminopropyltriethoxysilane (4 mL) was gradually added, with continuous stirring. The mixture was refluxed for 6 h. The silylated nanometer SiO2 was filtered off, washed with toluene and ethanol and dried at 60 °C for 3 h. Reaction mixture was filtered under vaccum and the chemically modified nanoparticles were stored in dessicator.
General procedure
Aliquots of cadmium sulphate containing 0.5 μg of Hg(II) was prepared and pH was adjusted to the 6.8 with 0.2 M sodium tetraborate/boric acid buffer, 35 mg of SiO2-APTMS particles were added, and the mixture was shaken vigorously for 15 min to facilitate adsorption of metal ion onto the adsorbent. 4 mL of 2 M hydrochloric acid was used for elution of Hg(II) from the adsorbent. Then, eluent was filtered and was determined by standard spectrophotometric method.
Results and discussion
Synthesis of silica nanoparticles by Sol–gel method
Bead size and the dispersity of the particle population depend strongly on the concentrations of alkoxysilane, ammonia and water. Temperature is also a controlling factor in the synthesis. A number of different approaches were investigated and it was found that the desired 100 nm spherical silica particles could be reproducibly prepared by the following procedure.
250 mL of methanol and 250 mL ammonium hydroxide (SG = 0.88) were mixed in a 1000 mL conical flask for 5 min using a magnetic stirrer. 5 mL of tetraethoxysilane was slowly added, whilst continuing the gentle stirring. The reaction was left to proceed at 20 °C for 1 h. The product was then centrifuged for 60 min at 3000 rpm at temperature of −4 °C and the supernatant was removed. The silica nanoparticles were rinsed with methanol several times, discarding the solvent at each stage after centrifugation. The silica nanoparticles were dried under vacuum and stored in a dry vial.
Modification of SiO2 nanoparticles
Nanometer SiO2 (1 g) was dispersed into dry toluene (30 mL) in a 250 mL flask, and then 3-aminopropyltrimethoxysilane (4 mL) was gradually added, with continuous stirring. The mixture was refluxed for 6 h. The silylated nanometer SiO2 was filtered off, first washed with toluene and then with ethanol and dried at 60 °C for 3 h and stored in a dry vial.
Characterization of SiO2-APTMS modified nanometer sized SiO2
The modification of nanometer-sized material is usually required to prevent a conglomeration of particles and to improve its consistency in relation to other materials, such as organic polymers (Stober et al. 1968; Toral et al. 2004; Silverstein and Morrill 1974). In addition, for the purpose of separation, the modification of nanometer-sized materials can improve the selectivity of nanometer-sized materials towards metal ions, organosiloxane is the most often used modifiers, in which one side is linked with the inorganic nanometer-sized materials, and the exposed end is the analytical functional group. The modified nanometer SiO2-APTMS was characterized by FT-IR and SEM.
Determination of amino groups on the silica surface
The method is based on the reaction between primary amines and ninhydrin, which produces a complex purple compound, known as Ruhemann’s purple, which absorbs strongly at 570 nm (ε = 22,000 mol L−1 cm−1).
Scanning electron microscopy (SEM)
The average diameter of the nanoparticles SiO2 and SiO2-APTMS was 100 nm and 1 μm confirmed by SEM. Figures 1a, b and 2a, b reveals the average size as well as the morphology of SiO2 nanoparticles, SiO2-APTMS and respectively.
FT-IR Spectrum Analysis (Abdouss and Mousavi-shoushtari 2012)
The main absorption peaks of nanometer SiO2 (3448.0, 1642.5, 1404, 1070.2, 964.2, 798.8 cm−1) are in agreement with standard spectrum of SiO2. It reveals that peak at 2907.2 cm−1 is due to C–H stretching, peak at 1641.3 cm−1 is due to N–H stretching and peak at 1093.5 cm−1 C–O stretching in 3-aminopropyltrimethoxysilane Fig. 3.
Determination of amino groups on the silica surface
The method is based on the reaction between primary amines and ninhydrin, which produces a complex purple compound, known as Ruhemann’s purple, which absorbs strongly at 570 nm (ε = 22,000 mol L−1 cm−1).
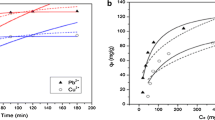
Adsorption isotherm study
To investigate the relationship between the adsorbed species and their equilibrium concentrations, the data generated from the column studies were fitted to Langmuir, Temkin and Frendlich isotherm models.
Langmuir isotherm
The Langmuir isotherm is applicable to adsorption on homogenous surfaces. According to this isotherm there are a constant number of available sites on the adsorbent surface. The linear form of this isotherm can be expressed as follows:
where b is a constant that increase with increasing molecular size and q max is the amount adsorbed to form a complete monolayer on the surface and C e is the equilibrium concentration of the solute. The values of Langmuir constants q max and b were calculated from slope and intercepts, respectively, of plots of \( \frac{{C_{\text{e}} }}{\frac{X}{M}} \) versus C e. The values of q max and b for the removal of Hg(II) from aqueous solution were found to be 0.2565 mg g−1 and 0.101 L mg−1.
Freundlich isotherm
The Freundlich isotherm has also been widely used for many years to model analyte adsorption at equilibrium. The isotherm model describes the equilibrium on heterogeneous surfaces and hence does not assume a monolayer capacity.
Temkin isotherm
The Temkin isotherm model was chosen to evaluate the adsorption potentials of the adsorbent for analytes. The derviation of the Temkin isotherm indicates the multilayer adsorption on the adsorbent. The Temkin isotherm has generally been applied in the following form.
The Temkin isotherm can be expressed by following equation.
where C is the concentration of adsorbate in solution at equilibrium (mgL−1), X is the amount of metal adsorbed (mggm−1), m is the weight of adsorbent (mg) and A and B are constants related to adsorption capacity and intensity of adsorption. The generalized data pertaining to Hg(II) does not fit the Temkin isotherm as the isotherm constant obtained was B = 0.049 which is out of range (1–50). This represents that there is no possibility for multilayer adsorption to take place.
It is clear that adsorption isotherm at temperature 35 °C can be fitted well using these isotherm models. However, the Langmuir model is more suitable than Frendlich model to describe the adsorption isotherm, as reflected with correlation coefficients. It suggests that adsorbent is homogenous and the adsorption is monolayer, the free energy (ΔG 0) of Hg(II) onto SiO2-APTMS nanoparticles is calculated based on the adsorption isotherm.
where R is the universal gas constant; T (K) the 35 °C of the solution and b L the Langmuir constant. The value of ΔG 0 is 20.2 kJ mol−1. It is noted that the ΔG 0 value is negative, which indicate the feasibility and spontaneous adsorption of Hg(II) onto SiO2-APTMS nanoparticles. The enthalpy change (ΔH 0) and entropy change (ΔS 0) could not be established because a linear relationship of ln b L and 1/T in the Van’t Hoff equation could not established.
Effect of pH on enrichment recovery
The adsorption of Hg(II) on nanometer SiO2-APTMS was studied at different pH value (3.4–10.0) following the recommended procedure. The results of effect of pH on the recoveries of the metal ions were shown in Fig. 4. It can be seen that a quantitative recovery (≥95 %) was found for Hg(II) in the pH range of 6.5–10.0.
Batch mode
Batch studies to measure the performance of the SPNEs in removing Hg(II) metal ions from waste water were conducted. For batch experiments the removal efficiency were tested by varying the dosage of the adsorbent, concentration of the analyte solution, contact time and pH of the analyte solution. The pH of the solution was varied using the sodium hydroxide and hydrochloric acid. After the equilibrium time, the adsorbent was separated from the solution by centrifugation and then eluted with different concentrations of hydrochloric acid and determined by spectrophotometry. The amount of the analyte adsorbed per unit mass of the adsorbent was determined using the equation:
Effect of eluent concentration and volume
Since the adsorption of ferbam on nanometer SiO2-APTMS at pH ≤2 is found to be negligible, elution will be favored in acidic solution. Elution of Hg(II) from nanometer SiO2-APTMS extractant was investigated by using various concentrations of hydrochloric acid. It can be seen that quantitative recoveries (>95 %) of Hg(II) can be obtained using 4 mL of 2 M hydrochloric acid as eluent. Therefore, 4 mL of 2 M of hydrochloric acid was used as eluent in subsequent experiments. The results of effect of eluent concentration and volume are given in Tables 1 and 2.
Effect of nanometer SiO2-APTMS amount
To test the effect of amount of extractant on quantitative retention of analyte, different amount (5–50 mg) of nanometer SiO2-APTMS were added into the solution following the experimental method. Quantitative extraction of the Hg(II) was obtained in the range of 5–50 mg of nanometer SiO2-APTMS. 35 mg of nanometer SiO2-APTMS as extractant was found to be sufficient for further studies. The results are shown in Fig. 5.
Effect of shaking time
The adsorption of Hg(II) on 35 mg of nanometer SiO2-APTMS was studied for different shaking time (10–45 min). The results indicated that within 15 min the extraction percentage of Hg(II) >95 % was achieved.
Adsorption capacity (QS)
The adsorption capacity (Mahmoud and Gohar 2000) is an important factor as it determines how much adsorbent is quantitatively required to concentrate the analytes from a given solution. A breakthrough curve was obtained by plotting the concentration (mg L−1) versus the μmol of Hg(II) adsorbed per gram. From the breakthrough curve the amount of modified nanometer SiO2-APTMS for Hg(II) was found to be 181.42 µmol g−1 at pH 6.8, respectively. The results are shown in Fig. 6.
Effect of sample volume
To explore the possibility of concentrating low concentration of analytes from large volumes, the effect of sample volume on the retention of metal ions was also investigated. For this purpose 50, 100, 150, 200, 250, 300 and 400 mL of the sample solutions containing 1.0 µg Hg(II) was shaken, quantitative recoveries (>95 %) were obtained for sample volume of ≤300 mL for Hg(II). The results are given in Fig. 7.
Effect of coexisting ions
The effect of common coexisting ions on the sorption of Hg(II) was investigated. In these experiments, a solution of 1.0 μg mL−1 of Hg(II) that contains the added interfering ion was analyzed according to the recommended procedure. The tolerance of coexisting ions defined as the largest amount making the recoveries of Cd(II) less than 90 %. In the determination of Hg(II), interfering ions like Cd(II), Cu(II) Fe(II) and Fe(III), were masked with 1 mL of 5 % of sodium fluoride solution and Bi(II), Pb(II), Co(II) and Ni(II) were masked with 2 mL of 1 M sodium citrate. Besides, a lot of anions NO3 −, SO4 2−, S2O3 2−, C2O4 2−, H2PO4 −, I−, CO3 2−and Cl− etc, did not interfere in preconcentration and determination of Hg(II).
Stability and reusability of adsorbent
The reusability and stability of the adsorbent was investigated. The capacity of the modified adsorbent was found to be apparently constant (less than 3 %) after the repeated use of more than four cycles of sorption and desorption of the target analytes.
Salting out effect
Addition of an electrolyte can reduce the extraction time by increasing the ionic strength and reducing analyte solubility. The electrolytes generally used for this purpose are NaCl, NaHCO3, K2SO4 and (NH4)2SO4. The salting out effect makes SPE more effective.
The influence of ionic strength on the extraction of Hg(II) in batch method was studied using sodium/or potassium chloride, bromide, sulphate, nitrate, chlorate (pH 2) with concentrations (0.1 mol L−1 to saturation). The results showed no effect on the extraction efficiency. These observations showed the specific tendency of SiO2-APTMS nanoparticles for Hg(II) and the possibility of using this method for separation of Hg(II) from highly saline solutions.
Analytical precision and detection limit
Under the selected conditions, three portions of Hg(II) standard solutions was enriched and analyzed simultaneously following the experimental method. The relative standard deviation (RSD) of the method was 1.2 % for the determination of 6.0 µg Hg(II) in 100 mL water samples respectively. The detection limit of this method for Hg(II) was 0.45 µg mL−1.
Applications
The developed method has been applied for the determination of trace Hg(II) in tap water, mineral water, waste water samples. For analysis, 200 mL of tap, mineral or waste water were studied by the developed method. The relative standard deviation (RSD %) and the detection limit of the method were 2.1 and 0.45 <gL−1, respectively.Statistical analysis of these results using Student’s t test showed that there was no significant difference between actual and found concentrations at 95 % confidence level.The results are given in Table 3.
Conclusions
The preconcentration method described by using 3-aminotripropyltrimethoxysilane anchored silica nanoparticles for the determination of Hg(II) in water samples has a good accuracy, repeatability and sensitivity. In this work, the advantages of nanoparticles as the sorbent including high adsorption capacity and facile extraction were combined with the advantages of spectrophotometric detection, including simplicity, speed, and inexpensiveness. Compared to other methods (especially complex hyphenated systems) the method had low detection limit and good precision (Abdouss and Mousavi-shoushtari 2012; Filho et al. 2005; Mahmoud and Gohar 2000; Newton et al. 2006; Fan et al. 2007; Sanchezrojas and De Torres 1998). Moreover, the method was simple and fast. The results of comparison of preconcentration factor are given in Table 4.
References
Abdouss M, Mousavi-shoushtari A (2012) Fabrication of chelating diethylenetriaminated pan micro and nano fibers for heavy metal removal. J Appl Polym Sci 18:27–34
Alonso EV, De Torres AG, Pavon JMC (2001) Flow injection online electrothermal atomic absorption spectrometry. Talanta 55:219–232
Anezaki K, Chen XZ, Ogasawara T, Nukatsuka I, Ohzeki K (1998) Determination of cadmium and lead in tap water by graphite-furnace atomic absorption spectrometry after preconcentration on a finely divided ino-exchange resin as the pyrrolidinedithiocarbamate complexs. Anal Sci 14:523–527
Bruzzoniti MC, Sarzanini C, Mentassi EJ (2000) Preconcentration of contaminants in water analysis. J Chromatogr A 902:289–309
Cao GH, Fang ZL (1999) Trends of flow injection samples treatment approaching the new millennium. Anal Chim Acta 360:233–247
Fan J, Lou C, Peng C, Peng P (2007) Preparation of xylenol orange functionalized silica gel aas a selective solid phase extractor and its applications for preconcentration—separation of mercury from water. J Hazard Mater 145:323–330
Filho NL, Carmo DR, Rosa AH (2005) Preconcentration and determination of mercury(II) at a chemically modified electrode containing 3-(2-thioimidazolyl) propyl silica gel. Anal sci 21:1359–1363
Henglein A (1989) Small-particle research: physiochemical properties of extremely small colloidal metal and semiconductor particles. Chem Rev 89:1861–1873
Hiraide M, Wasawa JI, Kawaguchi H (1997) Collection of trace metals complexed with ammonia pyrrolidinecarbamate on surfactant-coated alumina sorbents. Talanta 44:231–237
Hosono E, Fujihara S, Kimura T, Imai H (2004) Non-basic solution routes to prepare ZnO nanoparticles. J Sol Gel Sci Technol 29:71–79
Karadjova I (1995) Determination of Cd, Co, Cr, Cu, Fe, Mn, Ni and Pb in natural waters alkali and alkaline earth salts by electrochemical atomic absorption spectrometry after preconcentration by column solid phase extraction. Mikrochim Acta 130:185–190
Liska I (1993) On-line versus off-line solid-phase extraction in the determination of organic contaminants in water: advantages and limitations. J Chromatogr A 655:163–176
Mahmoud ME, Gohar GA (2000) Silica gel-immobilized dithioacetal derivatives as potential solid phase extractor for mercury(II). Talanta 51:77–78
Martinez D, Cugat MJ, Borrull F, Callul M (2000) Solid phase extraction couplingto capillary electrophoresis with emphasis on environmental analysis. J Chromatogr A 902:65–89
Newton DF, Laercio C, Rosandre H (2006) Preparation of a silica gel modified with 2-amino-1,3,4-thiadiazole for adsorption of metal ions and electrolytical applications. J Braz Chem Soc 17:437–481
Okuyama K, Lenggoro IW (2003) Preparation of nanoparticles via spray route. Chem Eng Sci 58:537–547
Panigrahi S, Kundu S, Ghosh S, Nath S, Pal T (2004) General method of syhtesis for metal nanoparticles. J Nanopart Res 6:411–414
Qufen H, Yang G, Yang J, Yin J (2002) Study on determination of iron, cobalt, nickel, copper, zinc and manganese in drinking water by solid-phase extraction and RP-HPLC with 2-(2-quinoliylazo)-5-dimethyl aminophenol. J Environ Monit 4:956–959
Ragai J, Selim ST (1987) Ion-exchange and surface properties of titania gels from Ti(III) solutions. J Colloid Interface Sci 115:139–146
Sanchezrojas F, De Torres AG (1998) Use of flow injection cold vapour generation and preconcentration on silica functionalized with methylthiosalicylate for determination of mercury in biological samples and sea-water by inductively coupled plasma atomic emission spectrometry. J Anal At Spectrom 13:1167–1171
Sarkar S, Cara PW, Mcneff CV, Subramanian A (2003) Charaterization and optimization of a chromatographic process based on ethlenediammine-N,N,N’,N’-tetra-(methylphosphonic)acid modified zirconia particles. J Chromatogr B 790:143–152
Schmidt H (2001) Nanoparticles by chemical synthesis processing to materials and innovative applications. Appl Organometal Chem 15:331–343
Shar GA, Soomaro GA (2004) Spectrophotometric determination of cobalt(II), nickel(II) and copper(II) with 1-(2-pyridylazo)-2-naphthol in micellar medium. Nucleus 41:77–80
Silverstein RM, Morrill TC (1974) Spectrometric identification of organic compounds, 3rd edn. Wiley, New York, p 115
Stober W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica sphere in the micron size range. J Colloid Interface Sci 26:62–69
Toral MI, Lara N, Narvaez J, Richter P (2004) Screening of some transition metal ions and quantitative determination of copper, cadmium and zinc by solid phase derivative spectrophotometry. J Chile Soc 49:163–169
Yaman M (1999) Determination of cadmium and lead in human urine by STAT-FAAS after enrichment on activated carbon. J Anal At Spectrom 14:275–278
Yang D, Cheng XJ, Liu YW, Wiang S (2004) Synthesis of efficiency of a spherical macroporous epoxy polyamide chelating resin for preconcentration and separating trace noble metal ions. Microchim Acta 147:219–223
Zolotov YA, Maksimova IM, Morosanova EI, Velikorodxy AA (1995) On-line coated columns for the spectrophotometric determination of metals by continous flow analysis. Anal Chim Acta 308:378–385
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kaur, A. Applications of organo-silica nanocomposites for SPNE of Hg(II). Appl Nanosci 6, 183–190 (2016). https://doi.org/10.1007/s13204-015-0413-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-015-0413-y