Abstract
An efficient and new magnetic nanoadsorbent photocatalyst was fabricated by co-precipitation technique. This research focuses on understanding metal removal process and developing a cost-effective technology for treatment of heavy metal-contaminated industrial wastewater. In this investigation, magnetic nanoadsorbent has been employed for the removal of Zn(II) ions from aqueous solutions by a batch adsorption technique. The adsorption equilibrium data fitted very well to Langmuir and Freundlich adsorption isotherm models. The thermodynamics of Zn(II) ions adsorption onto the magnetic nanoadsorbents indicated that the adsorption was spontaneous, endothermic and physical in nature. Surface morphology of magnetic nanoadsorbent by scanning electron microscopy (SEM) and elemental analysis by EDX technique. The structural and photocatalytic properties of magnetic nanoadsorbent were characterized using X-ray diffraction (XRD) and FTIR techniques. Also, the magnetic properties of synthesized magnetic nanoadsorbent were determined by vibrating spinning magnetometer (VSM).
Similar content being viewed by others
Introduction
Nanotechnology has been considered as one of the most important advancements in science and technology. Its essence is the ability to fabricate and engineer the materials and systems with the desired structures and functionalities using the nanosized building blocks (Siegel et al. 1999). Nanoparticles are one of the important building blocks in fabrication of nanomaterials. Their basic properties, extremely small size and high surface area to volume ratio, provide better kinetics for the adsorption of metal ions from aqueous solutions. However, for such an application, it is necessary to use a method of purification that does not generate secondary waste and involves materials that can be recycled and easily used on an industrial scale (Ngomsik et al. 2005).
In most industrial countries, heavy polluters like metallurgy industries have been forced to improve their techniques and to radically reduce the discharge of toxic trace metals to the atmosphere and water. Over the same period, the production, transformation and use of these metals in society, sometimes referred to as metal metabolism, have increased (Bergbäck 1992).
The most severe medical and ecological effects have been observed in areas with intense mining and metallurgy industries. During processing of the ore, the toxic metals, which often naturally occur at very low concentrations (trace metals), are released to the environment, resulting in high concentrations at a local scale. As a consequence, until the 1980s such metal pollution was generally considered to be a local problem mainly occurring around an easily identified point source. However, even on a global scale, anthropogenic activities are the most important source of the potentially toxic metals found in the environment (Campbell et al. 1983). Around 15 times more cadmium (Cd), 100 times more lead (Pb), 13 times more copper (Cu) and 21 times more zinc (Zn) are emitted to the atmosphere by human activities than by natural processes (Ross 1994). Therefore, for the removal of heavy metal, activated carbon has undoubtedly been the most popular and widely used adsorbent in wastewater treatment applications throughout the world. In spite of its prolific use, activated carbon remains an expensive material since higher the quality of activated carbon, the greater its cost. Activated carbon also requires complexing agents to improve its removal performance for inorganic matters.
Therefore, this situation makes it no longer attractive to be widely used in small-scale industries because of cost inefficiency. Due to the problems mentioned previously, research interest into the production of alternative adsorbents to replace the costly activated carbon has intensified in recent years. Attention has been focused on the various adsorbents, which have metal-binding capacities and are able to remove unwanted heavy metals from contaminated water at low cost.
With the rapid development of nanotechnology, magnetic nanoparticles are currently studied widely. Superparamagnetic iron oxide (Fe3O4) nanoparticles have attracted researchers from various fields such as physics, medicine, biology, and materials science due to their multifunctional properties such as small size, superparamagnetism, and low toxicity (Gu et al. 2006; Katz and Willner 2003). Several methods have been developed to synthesize magnetic Fe3O4 nanoparticles: (1) co-precipitation of ferrous (Fe2+) and ferric (Fe3+) aqueous solution in the presence of a base (Kang et al. 1996); (2) thermal decomposition of an iron complex (Woo et al. 2004); (3) by a sonochemical approach (Suslick et al. 1996). However, Fe3O4 nanoparticles tend to aggregate due to strong magnetic dipole–dipole attractions between particles. So, stabilizers such as surfactants, oxide or polymeric compounds (especially biocompatible polymer) with some specific functional groups have been used to modify these particles to increase the stability (Santra et al. 2001; Mikhaylova et al. 2004; Wa et al. 2006; Kim et al. 2006).
The difficulty in preparing Fe3O4 magnetic nanoparticles by chemical co-precipitation is the tendency of agglomeration of particles because of extremely small particle size leading to great specific surface area and high surface energy, consideration of effect of alkali, emulsifier, and reaction temperature are the decisions making factors of final product (Zhao et al. 2008).
In present study, the methods such as high-temperature decomposition of organic precursors, microemulsions, polyols, other solution techniques are require high temperature, sophisticated instruments, high-cost precursor compounds and time consuming. Therefore, Fe3O4 magnetic nanoparticles synthesized by co-precipitation method. The co-precipitation method is simple and gives effective result within 15 min. Also, no sophisticated instrumentation and assembly required for synthesis of Fe3O4 nanoparticles. The chemical co-precipitation reaction is eco-friendly and cost effective because only two iron precursors and dilute solution of NH4OH were required in present research article. During the fabrication of Fe3O4 nanoparticles there is no harmful by-product formation takes place, very small amount of heat energy required for the rise in temperature up to 70 °C, therefore, it is an energy-efficient reaction.
The synthesized Fe3O4 employed for the treatment of aqueous solution of Zn(II) ions. These treatability studies focus on the synthesis of Fe3O4 nanoparticles and their application in effective removal of Zn(II) ions from industrial and sewage wastewater by batch adsorption process. Different parametric studies were also carried out for determining optimum factors of corresponding parameters; the optimum pH is 5.5. This new technique developed is beneficial to water treatment and water purification processes.
Experimental
Materials and methods
Stock metal solutions were prepared by dissolving stoichiometric amount of Zn(NO3)2 in double-distilled water (DDW). All the reagents were used are A.R grade and obtained from Loba Chemie Industries Ltd. Mumbai.
Batch adsorption experiments
The experiments were conducted by introducing 60 mg (2 g L−1) of magnetic nanoadsorbent into a series of conical flasks 100 ml containing 30 mL of solutions having a metal concentration of 10 mg L−1. The glass conical flasks containing reaction mixture were agitated on magnetic stirrer at 25 °C for 1.30 h. The adsorbent treated colloidal solution was separated from the metal solution by centrifugation at 3,000 rpm for 20 min. The concentration of the metal in the remaining solution (supernatant liquid) was measured by double-beam spectrophotometer at systronics-2203.
Fe3O4 nanoparticles were prepared by co-precipitation method with a ferrous complex in presence of NH4OH. First, FeCl2·4H2O and FeCl3·6H2O [Fe2+:Fe3+ = 1:2] were dissolved in about 50 ml de-ionized water, and stirring this solution under strong ultrasonic agitation while heating solution to 70 °C. Next, this iron solution source was added dropwise into NH4OH under strong ultrasonic agitation for 30 min, and bubbling N2 gas.
The chemical reaction of Fe3O4 precipitation is expected as follows:
Black Fe3O4 particles were decanted by permanent magnet and cleaned by de-ionized water several times.
The magnetic properties of Fe3O4 nanoparticles can be observed visually. Photo image shows the iron oxide Fe3O4 nanoparticles in a vial as a magnet is brought in contact with the side of the vial. The Fe3O4 nanoparticles are attracted to the wall of the vial by the magnetic field.
This experimental study influences that these magnetic nanoparticles will be easily removed from aqueous solution after use by a permanent magnet as shown in the following photo image.
Result and discussion
The influences of various parameters have been discussed below. These parameters have been studied in the literature for different other adsorbent and contaminants but the results obtained and presented for magnetic nanoadsorbent in intact and pre-treated forms used for treating aqueous solutions containing Zn(II) ion different concentrations are presented here for treatment of industrial wastewater containing heavy metals.
Effect of pH
Experiments were conducted with intact and modified magnetic nanoadsorbent for Zn(II) adsorption in batch systems. The adsorption characteristic of metal adsorption at various pH values in the range was examined. Although all the experiments have been carried out for all intact and pre-treated magnetic nanoadsorbent, for this reason only the optimum modifier has been plotted. As shown in Fig. 1, the adsorption of Zn(II) metals ion is highly pH dependent and adsorption of Zinc is highest at pH values of 5.5 and then decreases as the pH increases.

Effect of adsorbent dose
Magnetic nanoadsorbent has a great influence on the adsorption process and determines the potential of removal of Zn(II) ions through the number of binding sites available to remove metal ions at a specified metal initial concentration. The effect of Magnetic nanoadsorbent on Zn(II) ion removal is indicated in Fig. 2. At equilibrium, capacity of Zn(II) ion removal decreases with an increase in magnetic nanoadsorbent dosage from 0.5 to 5 mg. This decrease can be due to the concentration gradient.
Effect of contact time
As the adsorption process proceeds, the sorbent reaches the saturation state and then the sorbed solute tends to desorb back into the solution. Eventually, the rates of adsorption and desorption will be equal at equilibrium. When the system reaches sorption equilibrium, no further net adsorption occurs. The time at which adsorption equilibrium occurs was determined (Sari and Tuzen 2008). In industry, this time is very important for the process optimization. The adsorption rate tests were performed on an equilibrium batch basis. The adsorbent was kept in contact with the metal-bearing solution for different times (25, 50, 75 min, 1, 2, 4, 8 and 16 h). From the experimental data represented in Fig. 3, the process of adsorption reaches the equilibrium state after approximately 2.5 h of contact. The reaction rate is rather fast at first and 80 % of total removal of Zn(II) ions of metals occurs in the first 55 min and thereafter it proceeds at a lower rate and finally no further significant adsorption is noted beyond 1.5 h. The very fast sorption and settling of the contact time make this material suitable for continuous flow water treatment systems.
Effect of initial metal concentration
The adsorption of Zn(II) ions was tested at lower concentrations because of their hazardous and toxic effect. Figure 4 indicates that the metal sorption capacity increases with an increase in the initial metal ion concentration. This is due to an increase in the initial ion concentration providing a larger driving force to overcome all mass transfer resistances between the solid and the aqueous phase, thus resulting in higher metal ion adsorption at initial stages. As concentration of Zn(II) ions in solution increases there is no further adsorption because of blocking of surface area of adsorbent. Similar results have been reported (Sari and Tuzen 2010; Riaz et al. 2009; Nasuha et al. 2010).
Result and discussion
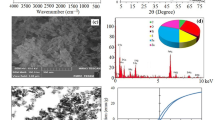
SEM analysis
The scanning electron microscopy (SEM) analysis shows the morphological structure of the material. In this result, there is distinct adsorption of Zn(II) ions on magnetic nanoadsorbent as Figs. 5a, 6a before adsorption show fine highly uniform nanosized surface area and Figs. 5b, 6b show change in uniform structure after adsorption of Zn(II) ions on Fe3O4 iron oxide nanoparticles. Adsorbed particles such as Zn(II) ions as well as the iron oxide particles are seen after adsorption. The iron oxide Fe3O4 nanoparticles exhibit magnetic behavior, creating more negative charges. The positively charged Zn(II) ions will be electrostatically attracted to the Fe3O4 nanoparticles. This is depicted by more particles being adsorbed in Figs. 5b, 6b. The nanosized structure of adsorbent has large surface area, which shows sufficient removal of Zn(II) ions from the aqueous solution.
XRD analysis
X-ray diffraction analysis of material gives the information of planes and size of synthesized material. In this study, magnetic nanoadsorbent shows the 2θ adsorption peaks in Fig. 7 at 30.2°, 35.5°, 42.8°, 52.8°, 57.6°, 62.8°, representing corresponding planes at 220, 311, 400, 422, 551 and 440, respectively. This clearly indicates that synthesized material Iron oxide magnetic nanoadsorbent is nanosized by calculating using Scherer formula Eq. (1). The data revealed that the magnetic nanoadsorbent is pure Fe3O4. The nanoadsorbent has large surface area, their unique size-dependent properties make these nanomaterials superior and indispensable in many areas of human activities such as medicine, environment, electronic, automotives, cosmetics. Here, magnetic nanomaterial holds the promise for producing better with less input energy or materials. Also, other application is developing specific drug delivery system and lab on a chip-based diagnostic for a minimal invasive medicine, improving information and communication through smaller and more powerful electronic devices.
EDX analysis
The EDX analysis shows in Fig. 8 the elemental composition of the synthesized material. In this research study, the synthesized material contains 61 % of oxygen, 37 % of iron and 2 % zinc as shown in Table 1. This clearly indicates that magnetic nanoadsorbent was useful for the removal of Zn(II) ions from aqueous solution.
FTIR analysis
The FTIR spectrum of synthesized magnetic material is of iron oxide magnetic nanoadsorbent. Figure 9 shows blue shift and characteristic absorption bands at 589 cm−1 of the Fe–O bond. Previously, it was reported that the characteristic absorption band of Fe–O bond of bulk Fe3O4 was at 599 and 375 cm−1 (Waldron 1995). However, when the size of Fe3O4 particles was reduced to nanoscale dimensions, the surface bond force constant increased due to the effect of finite size of nanoparticles, in which the breaking of large number of bonds for surface atoms resulted in the rearrangement of non-localized electrons on the particle surface (Ma et al. 2003).
VSM analysis
The most common measurement method employed for hysteresis loop determinations at ambient temperature is the vibrating sample magnetometer (VSM). This system is used to measure the magnetic properties of materials as a function of magnetic field, temperature, and time. Also, VSM analysis gives the idea about whether the magnetization is parallel or perpendicular to the plane defined by the substrate. In present result, VSM characterization carried out at 0–10,000 gauss and observed magnetization (Ms) about 59 emu gm−1 in Fe3O4 nanoparticles. Based on Fig. 10, VSM curve at room temperatures and magnetic behavior of the magnetic nanoadsorbent can be identified. For example, at room temperature, Fe3O4 particles show hysteresis loop feature, indicating that the magnetic Fe3O4 particles are paramagnetic; from this, it is clear that the magnetic moment aligns perpendicular to the applied magnetic field (H). Magnetic properties of the Fe3O4 particles depend upon crystalline nature of the nanoparticles. We can optimize the magnetic property by increasing crystallinity of the Fe3O4 nanoparticles. Also, from the plateau part of the VSM curve, saturation magnetization (Ms) can be determined. In present characterization, most important is that the Fe3O4 particles materials should possess sufficient magnetic properties for use in practical applications and it is very useful in the present study.
Kinetic study
Kinetic study provides valuable information about the mechanism of adsorption and subsequently investigation of the controlling mechanism of the adsorption process as either mass transfer or chemical reaction to obtain the optimum operating conditions for industrial-scale batch processes (Alyuz and Veli 2009). In batch systems, adsorption kinetics is described by a number of models based on adsorption equilibrium such as the pseudo-first-order and the pseudo-second-order kinetic models. The linearized pseudo-first-order kinetic model takes the following form of Eq. (2) (Chen et al. 2008; Basha and Murthy 2007).
where q t and q e are the amounts of metal adsorbed at time t and equilibrium, respectively, and K 1 (min−1) is the first-order reaction rate constant. The pseudo-second-order kinetic model considered in this study is given as (Chen et al. 2008; Basha and Murthy 2007) Eq. (3).
where K 2 (gmg−1 min−1) is the second-order reaction rate constant. The experimental data and the parameters of both models are tabulated in Table 2. It is obvious that the coefficient of correlation (R 2 = 0.99) for the pseudo-second-order kinetic model is higher in comparison with the pseudo-first-order kinetic model (R 2 = 0.97) for Zn(II) ion adsorption. The calculated value of q e for the pseudo-second-order kinetic model is very close to the experimental value. Similar experimental results indicate that the pseudo-second-order kinetic model fits the equilibrium data for heavy metal ion sorption on biomasses from aqueous solutions quite well (Chen et al. 2008; Sari and Tuzen 2008; Sari and Tuzen 2010). The comparison between adsorption rate constants, the estimated qe and the coefficients of correlation associated with the Lagergren pseudo-first-order and the pseudo-second-order kinetic models at 25 °C.
Thermodynamic studies
Thermodynamic studies are used to decipher any reaction in a better way. In the present studies also, thermodynamic studies were performed and the parameters, namely free energy change (∆G°), enthalpy (∆H°), and entropy (∆S°), were determined at 303, 313 and 323 K, respectively (shown in Table 3). Thermodynamic parameters were calculated using the following four equations Eq. (4)–(7).
K c is the equilibrium constant, and C ac and C e are the equilibrium concentration of metal ions on the adsorbent (mg L−1) and the equilibrium concentration of the metal ions in the solution (mg L−1), respectively. The values of K c increased as the temperature is increased, indicating the endothermic nature of the process of removal. The values of these parameters are given in Table 2. Positive value of entropy change ∆S° and enthalpy change ∆H° also indicate the endothermic nature of adsorption of Zn(II) in magnetic nanoadsorbent. It is noted that values of ∆G° decrease with increasing temperature.
Conclusion
-
The employed magnetic nanoadsorbent for Zn(II) ions removal was influenced by various parameters such as Initial pH, Initial Zn(II) ions concentration, contact time and adsorbent dose. The maximum adsorption Zn(II) ions on magnetic Fe3O4 nanoparticles occurred at pH 5.5.
-
The SEM analysis study of magnetic nanoadsorbent before and after treatment shows distinct removal of Zn(II) ions and adsorbent shows nanosized particles. Also, EDX study indicates the Zn(II) ions were clearly adsorbed from EDX analysis data.
-
The XRD result shows the different 2θ peaks, which show different planes and sizes of Fe3O4 nanoparticles calculated by Scherrer formula.
-
The VSM analysis of Fe3O4 nanoparticles shows sufficient magnetic properties.
-
Equilibrium and thermodynamic study of Zn(II) ion removal shows that the adsorption process is spontaneous and endothermic.
References
Alyuz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J Hazard Mater 167:482–488
Basha S, Murthy ZVP (2007) Kinetic and equilibrium models for biosorption of Cr(VI) on chemically modified seaweed, Cystoseira indica. Process Biochem 42:1521–1529
Bergbäck B (1992) Industrial metabolism. The emerging landscape of heavy metal immission in Sweden. Dissertion, Linköping studies in Arts and Science, p 76
Campbell PGC, Stokes PB and Galloway JH (1983) Effects on atmospheric deposition on the geochemical cycling and biological availability of metals. In: Proceedings of heavy metals in the environment, International Conference, Heidelberg, CEPConsultants, Edinburgh vol 2, pp 760–763
Chen Z, Ma W, Han M (2008) Biosorption of nickel and copper onto treated alga (Undaria pinnatifida): application of isotherm and kinetic models. J Hazard Mater 155:327–333
Gu HW, Xu KM, Xu CJ, Xu B (2006) Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem Commun 6:941–949
Kang YS, Risbud S, Rabolt JF, Stroeve P (1996) Synthesis and characterization of nanometer-size Fe3O4 and Fe2O3 particles. Chem Mater 8:2209–2212
Katz E, Willner I (2003) Magnetic control of electrocatalytic and bioelectrocatalytic processes. Angew Chem Int Ed 42:4576–4588
Kim DH, Lee SH, Im KH, Kim KN, Kim KM, Shim IB, Lee MH, Lee YK (2006) Surface-modified magnetite nanoparticles for hyperthermia: reparation, characterization, and cytotoxicity studies, Curr Appl Phys 6(S1):e242–e246
Ma M, Zhang Y, Yu W, Shen HY, Zhang HQ, Gu N (2003) Colloids Surf. Physicochem Eng Asp 212:219–226
Mikhaylova M, Kim DK, Bobrysheva N, Osmolowsky M, Semenov V, Tsakalakos T, Muhammed M (2004) Superparamagnetism of magnetite nanoparticles: dependence on surface modification. Langmuir 20:2472–2477
Nasuha N, Hameed BH, Mohd Din AT (2010) Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J Hazard Mater 175:126–132
Ngomsik A, Bee A, Draye M, Cote G, Cabuil V (2005) Magnetic nano and microparticles for metal removal and environmental applications: a review. C R Chimie 8:963–970
Riaz M, Nadeem R, Hanif MA, Ansari TM, Rehman KU (2009) Pb(II) biosorption from hazardous aqueous streams using Gossypium hirsutum (Cotton) waste biomass. J Hazard Mater 161:88–94
Ross S (1994) Sources and forms of potentially toxic metals in soil–plant systems. In: Ross S (ed) Toxic metals in soil–plant systems, Wiley, Chichester, pp 23–25
Santra S, Tapec R, Theodoropoulou N, Dobson J, Hebard A, Tan WH (2001) Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: the effect of nonionic surfactants. Langmuir 17:2900–2906
Sari A, Tuzen M (2008a) Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass. J Hazard Mater 152:302–308
Sari A, Tuzen M (2008b) Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. J Hazard Mater 160:349–355
Sari A, Tuzen M (2010) Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: equilibrium, thermodynamic and kineticstudies. Chem Eng J 158:200–206
Siegel RW, Hu E, Roco MC (1999) Nanostructure science and technology: a worldwide study. WTEC, Loyola College Kluwer Academic, Baltimore
Suslick KS, Fang M, Hyeon T (1996) Sonochemical synthesis of iron colloids. J Am Chem Soc 118:11960–11961
Wa SR, Huang JS, Yan HS (2006) Size-controlled preparation of magnetitenanoparticles in the presence of graft copolymers. J Mater Chem 16:298–303
Waldron RD (1995) Phys Rev 99:1727–1735
Woo K, Hong J, Choi S, Lee H, Ahn J, Kim CS, Lee SW, (2004) Easy synthesis and magnetic properties of iron oxide nanoparticles, Chem. Mater: 162814–3218
Zhao Y, Qui Z, Huang J (2008) Preparattion and analysis of Fe2O3 magnetic nanoparticles used as targeted-drug. Carr Chinese J chem Eng 16(3):451–455
Acknowledgments
Authors gratefully acknowledge Shri.G.H. Raisoni Doctoral Fellowship at North Maharashtra University, Jalgaon for Financial Support and North Maharashtra University, Jalgaon for SEM, EDX and XRD studies and thankful to Dept. of Physics, Pune University for VSM characterization. Authors are also thankful to the Principal G.T.P. College, Nandurbar for providing necessary laboratory facilities and FTIR characterization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Shirsath, D.S., Shirivastava, V.S. Adsorptive removal of heavy metals by magnetic nanoadsorbent: an equilibrium and thermodynamic study. Appl Nanosci 5, 927–935 (2015). https://doi.org/10.1007/s13204-014-0390-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0390-6