Abstract
Effect of LaCl3 concentration and annealing temperature on the diode ideality factor of LaF3/PS heterojunction has been investigated in this report. LaF3 layers have been deposited by a novel chemical bath deposition (CBD) technique. With this simple technique LaF3 produced as LaCl3 are made to react with hydrofluoric acid on the porous silicon (PS) substrate. This enables direct deposition of LaF3 on the pore walls of the PS leading to a successful passivation of PS. The compositions of the deposited LaF3 were confirmed by energy dispersive of X-ray analysis. The diode ideality factor increases with LaCl3 concentration and decreases with annealing temperature. Therefore, by changing the LaCl3 concentration and annealing temperature quality of the LaF3 layer on PS can be optimized. It was also seen that the Ag/LaF3/PS/Si/Ag structure showed the formation of Schottky diode with a threshold voltage of about 5.5 V. From the experimental results it can be concluded that lanthanum fluorides can be deposited into the pores as well as on the top of PS by the CBD technique, which provides the required passivation for PS. This passivation can enable the PS to be considered as an important material for photonics.
Similar content being viewed by others
Introduction
Recently, there has been increasing interest in semiconductor materials, which find applications in optoelectronic, photovoltaic industries and photoelectrochemical solar cell devices. Among these materials, LaF3 thin films appear to be promising candidates for many technological applications due to their stability, band gap energy(about 10.3 eV) (Pilvi et al. 2008) transparency and photoconductor behavior. A disadvantage of this material is the aging, that is, the slow spontaneous oxidation of porous silicon (PS) (Boukherroub et al. 2000). [PS can be considered as a silicon (Si) crystal having a network of voids in it (Andrea Edit Pap)]. This chemical conversation is slow and basically similar to the aging of Si wafer, i.e., a native oxide layer forms on the surface of the pores and the thickness of this oxide layer grows with time. Due to the aging effect, the structural, compositional, electrical and optical properties of PS show continuous change with storage time (Boukherroub et al. 2000). That is many of its properties, such as photoluminescence, are age dependent and unstable. Tischier et al. observed that the exposure of PS in different ambient results in a rather rapid decrement of photoluminescence (PL) intensity Yimin Fan. One possible way to reduce the aging effect could be “passivation” of PS. Passivation is defined as the process of forming a protective film on an active material surface to reduce the chemical reactivity of the surface and protect it against contamination and increase its stability by isolating the surface from chemical and electrical conditions in the environment. Over the years, many passivation methods, such as anodic oxidation and rapid thermal oxidation, have been attempted to improve the stability, as well as efficiency, of PS. However, these passivation methods always carry the danger of a total oxidation of the PS layer and of transforming it into SiO2. Because of various advantages of LaF3 like good moisture resistance (Hopkins et al. 1975), large bad gap (Pilvi et al. 2008), passivation of PS has been investigated for the first time in our previous articles (Abdul Al et al. 2012). By reacting LaCl3 with hydrofluoric acid (HF), LaF3 has been tried to deposit into the pores of PS surface with a goal of not to allow the PS sample to be oxidized during transportation and drying for passivation like other deposition techniques (Abdul Al et al. 2012). This article reports the influence of LaCl3 concentration and annealing temperature on the diode ideality factor of LaF3/PS structure. The ideality factor η is a measure of the conformity of the diode behavior to pure thermionic emission and is a dimensionless factor introduced to account the deviation from the thermionic emission diffusion theory.
Experimental
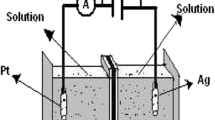
The PS has been prepared in the home made double-tank cell by anodic etching of silicon wafer shown in Fig. 1, (Halimaoui 1997). The anodic etching of Si was done in a 1:1 (for n-type silicon) solution of 48 % HF and absolute ethanol and for p-type it was 3:1. The LaF3 films with different LaCl3 concentrations and annealing temperatures were prepared. The same double-tank chamber in the upright position is used for LaF3 deposition (Fig. 2).
Just after the anodic etching, the etching solution was drained out and the fresh HF was introduced in the chamber to wash out any remaining etching solution on the chamber. After draining out the washing HF, solution of LaCl3 and 48 % HF were introduced simultaneously into the etching chamber to initiate the chemical reaction and produces LaF3 that deposits on the just prepared PS sample. Thus the PS is never exposed to the environment before passivation and this chemical bath deposition(CBD) method of LaF3 deposition should prevent the PS to be oxidized.
The forward bias lnI−V (where I represents current and V represents voltage) plots of different samples are drawn and from where the diode ideality factors are calculated. The diode ideality factor increases with LaCl3 concentration and decreases with annealing temperature.
Anodic etching was carried out using an electrolyte of HF (48 %) and ethanol (98 %) in 1:1 proportion under a constant current density of 15 mA/cm2 for 30 min at room temperature. The electrochemical anodization of Si wafer was done using a double-tank cell set-up (Abdul Al et al. 2012). The wafer was cut into pieces and these pieces of Si wafer were cleaned by successively immersing in acetone, ethanol and de-ionized water. The electrolyte consisted of HF:C2H5OH in the ratio of 1:1 by volume. A 100-W tungsten lamp was used for illumination from 15 cm distance. After 30 min anodization, the etching solution and back contact solution was drained out keeping the samples in the etching chamber (Halimaoui 1997). Fresh HF was then introduced in the chamber to wash out any remaining etching solution on the chamber. After draining out the HF that used for washing, 0.2, 0.4 or 0.6 solution of LaCl3 and 48 % HF were introduced simultaneously into the etching chamber through the “HF in” and “LaCl3 in” channels to do the chemical reaction. The chemical reaction that produces LaF3 is pretty simple, at room temperature, the addition of hydrofluoric acid to an aqueous solution of lanthanum chloride precipitates out lanthanum fluoride, LaF3 (Patnaik 2002). The formation of white precipitate (LaF3) confirmed the mechanism of film formation.
The solution inside the etching chamber was stirred for 10 s and resulting LaF3 crystals were allowed to passivate the PS layer for 4 min. After each cycle of reaction, the solution was drained out through the “Solution out” channel and a new solution was introduced into the chamber for the next deposition cycle. In this case, the deposition results from a chemical reaction in solution, which may involve the surface silicon atoms, and in this case, we will speak of chemical grafting of the surface, and why the reaction is limited to the formation of one monolayer (Herino 2000). The whole process was repeated to obtain various thicknesses of LaF3 on to PS. After completing the required cycle the wafer was removed from the chamber, rinsed with de-ionized water and dried in air at room temperature.
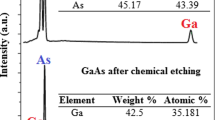
The compositional investigations have been done by the energy dispersive X-ray (EDX) spectroscopy. For current−voltage (I−V) characterization of the lanthanum fluoride deposited PS sample, silver (Ag) film was evaporated onto the front and backside of the sample in a small area using Edwards E-306A vacuum coating unit. Then the copper wires were connected onto the Ag layer, on both sides with silver paste. The arrangement for I−V characterization is shown in Fig. 3.
Results and discussion
The ideality factor is calculated from the slope of the linear region of the forward bias lnI−V plot and can be written as:
where q is the charge of electron, k is the Boltzmann constant, T is the absolute temperature and dV/d(lnI) is the slope of the linear region of the forward bias lnI−V plot.
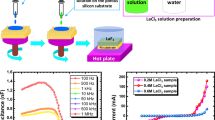
The chemical bath deposited LaF3 on PS produces a heterostructure system (LaF3/PS/Si). The forward bias lnI−V plots of different samples are shown in Fig. 4, 5 and 6 and the ideality factors of that samples are listed in Table 1.
From Table 1 it is clear that the diode ideality factors for different samples are different. The variations of diode ideality factors with LaCl3 concentration and annealing temperature are shown in Figs. 7 and 8, respectively.
From Figs. 7 and 8 it is clear that the diode ideality factor increases with LaCl3 concentration and decreases with annealing temperature.
In this report LaF3 was deposited on PS by CBD technique with a home made double-tank cell set-up. From the EDX data it was confirmed that the LaF3 was deposited on PS in the in situ technique. Later the influence of LaCl3 concentration and annealing temperature on the diode ideality factor of LaF3/PS structure has been investigated. The diode ideality factor increases with LaCl3 concentration and decreases with annealing temperature. It might be due to the reduction and increment of LaF3 layer thickness. It was also seen that the Ag/LaF3/PS/Si/Ag structure showed the formation of Schottky diode with a threshold voltage of about 5.5 V (Fig. 9). This might be due to the fact that annealing removes the oxygen from the PS and LaF3 crystals and rearranged the system, therefore the junction became stable.
Conclusions
Hence, from this research, it can be concluded that LaF3 can be efficiently deposited on PS by the CBD technique. The aim of the thesis was to investigate the influence of LaCl3 concentration and annealing temperature on the diode ideality factor of LaF3/PS structure. The EDX confirmed the deposition of LaF3 on PS.
From the I−V characteristics, it was observed that the annealed LaF3-deposited PS sample behaves like Schottky diode with threshold voltage approximately 5.5 V.
From these experimental results it can also be concluded that the passivating layer of LaF3 on PS can be optimized by the annealing temperature. This optimized layer of LaF3 can enable the PS to be an important material in electronic and optoelectronic device fabrication.
References
Abdul Al M, Hafijur R, Sinthia SM, Julkar N, Ismail ABM (2012) Passivation of porous silicon by LaF3 using a simple single-source chemical bath technique. Int J Mater Chem 2(4):111–115
Andrea Edit Pap, Faculty of Technology, University of oulu, P.O. Box 4000, FIN-90014 University of oulu Finland. (http://herkules.oulu.fi/isbn9514277759/)
Boukherroub R et al (2000) Thermal route for chemical modification and photoluminescence stabilization of porous silicon. Phys. Stat. Sol. A 182:117–121
Halimaoui A (1997) Porous silicon formation by anodisation In: Properties of porous silicon (ed). by L T Chanham. IEE INSPEC, The institution of electrical engineers, London, 12–13
Herino R (2000) Nanocomposite materials from porous silicon. Mater Sci Eng B 69–70:70–76
Hopkins RH, Hoffman RA, Kramer WE (1975) Appl Opt 14:2631
Patnaik P (2002) Handbook of inorganic chemicals. McGraw-Hill Professional, New York 448
Pilvi T, Puukilainen E, Arstila K, Leskela M, Ritala M (2008) “Atomic layer deposition of LaF3 thin films using La(thd)3 and TiF4 as precursors”. Che Vap Depos 14:85–91
Yimin Fan, School of Materials Science and Engineering, Shanghai University Shanghai 201 800, China
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hafijur Rahman, M., Ismail, A.B.M. Influence of LaCl3 concentration and annealing temperature on the diode ideality factor of LaF3/porous-silicon structure prepared by chemical bath deposition technique. Appl Nanosci 5, 645–649 (2015). https://doi.org/10.1007/s13204-014-0358-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0358-6