Abstract
Pure ZnO and 2 % Cr-doped ZnO nanoparticles were synthesized by sol–gel technique. Powder X-ray diffraction reveals that Cr incorporates into the ZnO crystal lattice without disturbing the original hexagonal structure. Transmission electron microscopy measurements show that the average size of these nanoparticles is in the range 20–25 nm. Pure ZnO nanoparticles exhibit diamagnetism while Cr-doped ZnO exhibit saturated hysteresis loop at room temperature indicating ferromagnetic behavior. Nitrogen co-doping along with Cr in ZnO shows enhanced ferromagnetism with small antiferromagnetism.
Similar content being viewed by others
Introduction
Dilute magnetic semiconductors (DMS) have attracted extensive curiosity as they have potential applications in the emerging field of spintronics (Wolf et al. 2001; Ohno 1998). The main challenge for use of DMS in practical devices is the achievement of Curie temperature above room temperature. Nanosized transition metal semiconducting materials are expected to bring global ferromagnetic order in the entire lattice at room temperature because of the exchange interactions between the spin of the dopant atoms and the carriers in the semiconductor host (Furdyna 1988). Dietl et al. 2000 also calculated theoretically that wide band-gap semiconductor (ZnO) having wurtzite structure that is doped with transition metals can be possible DMS material at room temperature. After these theoretical predictions, different research groups worldwide have started work on transition metal doped ZnO synthesis using different techniques (Sato and Katayama-Yoshida 2000, 2001; Ueda et al. 2001; Prellier et al. 2003). However, transition metal doping in DMS materials leads to some problems such as formation of secondary phases or clusters, and the mechanism of ferromagnetism in these materials is still unclear, making them undesirable for device applications. These DMS nanoparticles can be useful in ferrofluid and biomedical applications (Deka et al. 2004; Schneider et al. 2009).
Co-doped ZnO systems are the most studied DMS materials (Pearton et al. 2003). Few groups also have worked on Cr-doped ZnO as DMS. Ueda et al. 2001 investigated no ferromagnetism in Cr-doped ZnO thin films grown using pulsed laser deposition technique while RTFM is observed Cr-doped ZnO thin films grown using co-sputtering method (Roberts et al. 2005). Liu et al. 2007 also reported RTFM in Cr-doped ZnO thin films synthesized using co-sputtering technique. But they also observed paramagnetic behavior of Cr-doped ZnO nanoparticles synthesized using sol–gel method (Coey et al. 2005). Duan et al. 2010 reported systematically that light Cr doping in ZnO could be an effective way to achieve enhanced ferromagnetism. They used the auto-combustion method for the synthesis of Cr-doped ZnO nanoparticles and suggested the solubility limit for 3 % Cr in ZnO based on experimental investigations. Sato and Katayama-Yoshida 2000 and 2001 predicted theoretically that ferromagnetism in Cr-doped ZnO is more stable and energetically favorable that ferromagnetism in widely studied Co-doped ZnO. Li et al. 2009 calculated the optical properties of Cr-doped ZnO system using first principle calculations and suggested that the optical energy gap will increase with increase in Cr doping in ZnO. They also reported that the Cr doping would become a significant factor influencing the photocatalytic activity of ZnO and make it potential candidate for photo-electrochemical application.
Assadi et al. 2009 investigated the N co-doping in Mn:ZnO system using first principle density functional calculations and suggested strong and stable ferromagnetic order. Assadi et al. 2009 also predicted enhanced ferromagnetism in ZnO co-doped with Co and N. Yan et al. 2007 reported that N incorporation in Mn:ZnO thin films synthesized using chemical vapor deposition method, greatly enhanced the magnetic moment per Mn. Xu et al. 2006 reported ferromagnetism in N and Mn co-doped ZnO thin films grown using reactive magnetron sputtering and suggested bound magnetic polaron model for the observed magnetism. Different groups have observed high-TC ferromagnetism in ZnO:Mn films by doping group V elements (N, P, and As) as p-type dopants and suggested the activation of ferromagnetism to the holes induction by the doping of acceptors (Kittistved et al. 2005; Xu et al. 2006). Among group V acceptors, N has been considered to be a potential acceptor for making p-type ZnO (Zhang et al. 2001; Kaminska et al. 2005). So we have chosen 2 % Cr doping in ZnO and then studied the effect of N co-doping in the same. In this paper, we reported the synthesis of N and Cr co-doped ZnO nanoparticles by sol–gel technique. Their structural and magnetic properties have also been studied systematically.
Experimental details
Cr-doped ZnO nanoparticles were synthesized by sol–gel technique. In this study, Zinc acetate dihydrate, 2-methoxethanol and monoethanolamine (MEA) were used as the starting materials, solvent and stabilizer, respectively, and chromium acetate, ammonium acetate as the source of dopant material. Zinc acetate dehydrate, chromium acetate and ammonium acetate were first dissolved in 2-methoxyethanol. The molar ratios of MEA to zinc acetate dehydrate was maintained at 1.0. Then ammonium acetate solution was added dropwise to chromium acetate (0.02) for nitrogen co-doping. The resulting mixture was stirred at 60 °C for 2 h. Finally, a clear and transparent homogeneous solution was formed. The total concentration of solution was kept at 0.5 mol/L. The sol was aged for 24 h at room temperature and dried in a furnace at 500 °C for 1 h. The dry samples were crushed in pestle mortar to get the fine powder which was again annealed at 500 °C for 1 h.
The structural properties of N and Cr co-doped ZnO nanoparticles were investigated using “Panalytical Xpert Diffractometer”. The particle size determination and structure confirmations were made using a FEI G2 High Resolution Transition Electron Microscopy (HR-TEM) coupled with an energy dispersive X-ray analysis (EDX) accessory. Magnetic measurements were made using a quantum design vibrating sample magnetometer (VSM).
Results and discussion
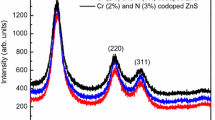
Rietveld refined X-ray diffraction (XRD) patterns of N and Cr-doped ZnO samples are shown in Fig. 1 in which all peaks are indexed in the wurtzite type hexagonal structure with space group P63mc (space group no. 186). Parameters Rp (profile fitting R-value), Rwp (weighted profile R-value) and χ2 (goodness-of-fit quality factor) observed after the last cycle of refinement for all samples are presented in Table 1. Small values of χ2 and profile parameters (Rp and Rwp) recommend that derived samples are of good quality and refinements are valuable.
On comparing with standard sample as per JCPDS card JCPDS-36-1451, the XRD and 2θ values are fairly in good agreement. It reveals that as prepared nanocrystals have a wurtzite (hexagonal) structure for the as prepared nanoparticles. Broadening of the diffraction peaks indicates the nanocrystalline nature of the ZnO NPs prepared. It is seen from the XRD patterns that the peaks of ZnO shift toward higher angles with Cr doping in ZnO. Such shifts of the XRD peaks reveal a lattice contraction due to the Cr substitution for Zn in the ZnO. This is because the radius of Cr ion (0.63 Å) is less than the ionic radius of Zn (0.74 Å) (Duan et al. 2010). But XRD peaks shift toward lower angles with N co-doping along with Cr in ZnO. Crystalline structure of the samples does not change but the quality spoils with the substitution of O by N, because the ionic radii of N (1.71 Å) is larger than that of O (1.40 Å) (Lee et al. 2010). The mean crystallite size in the samples was estimated using Scherrer formula.
where D is the mean grain size, k is a geometric factor, k = 0.89, λ = 0.154056 nm is the X-ray wavelength, β is the FWHM of diffraction peak and θ is the diffraction angle. The size observed is in the range of 15–18 nm.
The local chemical compositions of the samples were characterized by energy dispersive X-ray spectroscopy (EDS). Energy dispersive X-ray spectroscopy was performed at several different locations in the same nanoparticle, resulting in the identification of similar chemical compositions, which suggests a fairly uniform distribution of the dopant ions. Figure 2a and b show the HR-TEM micrographs along with EDX spectrum for 2 % Cr-doped ZnO and 3 % N and 2 % Cr co-doped ZnO nanoparticles, respectively. From transmission electron microscopy (TEM) micrographs it can be easily depicted that the size of the particles is in the range of 20–25 nm for both 2 % Cr-doped ZnO and 3 % N and 2 % Cr co-doped ZnO, which are slightly greater than the size estimated from Scherrer formula. Lattice fringes observed in both cases depicts the crystalline nature of the nanoparticles. The EDX spectrum clearly indicates the presence of dopants Cr and N in ZnO matrix. It also shows the peaks for Cu and C which are basically coming because of the carbon coated Cu grids used for TEM.
In order to calculate the optical band gap (Eg,) from the absorption spectra (Fig. 3a), Tauc-plot studies of the variation of the absorption coefficient (α) with photon energy is used:
where Eg is the optical band gap and A is a constant.
Figure 3b shows the plot of (αhν)2 versus (hv) for the pure and doped ZnO samples. Band-gap values are estimated by extrapolating the linear portions of the plots onto the energy axis and these analyses show an apparent increase in Eg from 3.14 to 3.20 eV with an increase of Cr content from x = 0.00 to 0.02 at % and further increase is observed i.e. 3.24 and 3.29 eV for 1 and 3 % N co-doping in 2 % Cr-doped ZnO.
Semiconductor particles having size greater than ‘‘Bohr radius’’ are coming under weak confinement regime. Size is very important even in weak confinement regime (Pandiyarajan and Karthikeyan 2012). TEM micrographs depict that the prepared particles are in weak confinement regime. In the present study, N co-doping with Cr plays a decisive role in tuning the band gap. Band gap of the particle will increase with decrease in the particle size as per the following relation (Van Dijken et al. 2000):
Where, M = me + mh is the total mass of the e–h pair, ‘R’ is the radius of the nano-particle, ‘Eg’ is the bulk band-gap energy and ‘Eexc’ is exciton binding energy and ‘n’ is the quantum number. Tampo et al. 2007 has reported that the band gap has cubic relation with the oscillator strength and the oscillator strength increases with increase in Mg doping in ZnO. In case of N co-doping with Cr may also result in Busrten-Moss effect in ZnO and result in increase in band gap (Sernelius et al. 1988).
The magnetic behavior of pure and doped ZnO nanoparticles was investigated using VSM at room temperature using a magnetic field (H) in the range of −5 to +5 kOe. It is seen from the Fig. 4 that the pure ZnO sample shows diamagnetic behavior with small signature of ferromagnetism. ZnO is a diamagnetic compound by nature and Cr is antiferromagnetic. Small signature of ferromagnetism in pure ZnO nanoparticles may be due the surface defects (Sundaresan et al. 2006). The appearance of weak ferromagnetic state in Cr-doped ZnO nanoparticles has been predicted by the theoretical calculation of Katayama-Yoshida et al. 2007. Various impurity phases like Cr2O3, Cr3O4, and Cr metal are antiferromagnetic while CrO2 is the only ferromagnetic. In our system, we observed no such impurity phases as depicted from XRD patterns. So the possible origin of ferromagnetism is the ferromagnetic coupling of Cr incorporated in ZnO matrix. The magnetic behavior of II-VI DMS has been extensively studied and observed to result from indirect sp-d interactions between the TM ions, which could be either from super-exchange or double-exchange interactions (Duan et al. 2010). McNorton and MacLaren 2009 reported that super-exchange results in antiferromagnetism while double exchange leads to ferromagnetism for TMs with more than half filled d shell while can be either antiferromagnetic or ferromagnetic for TMs with less than half filled d shell. So for Cr-doped ZnO nanoparticles, the possibility is equal for either antiferromagnetic or ferromagnetic behavior for Cr3+ (3d3). Duan et al. 2010 explained the observed ferromagnetism in a simple way that the favored ferromagnetic super-exchange between Cr3+ takes place when a p-electron is transferred, the spin S on the TM is maximized, lowering the ground state energy in corroboration with Hund’s Rule. Kittilstved et al. 2006 reported that the major characteristic is universal to all of the models describing ferromagnetism in DMS is the strong electronic coupling between the magnetic ions and charge carriers at the Fermi level.
Assadi et al. 2009 reported the holes induction in the Mn:ZnO system with nitrogen co-doping. Yan et al. 2007 reported the first-principle calculations and suggest that the N substitution for the O site in Mn-doped ZnO can change the interaction of neighboring Mn–Mn pairs from antiferromagnetic to ferromagnetic, and accordingly, they observed that the effective magnetic moment per Mn is greatly enhanced for N co-doped Mn:ZnO thin films grown using inductively coupled plasma enhanced chemical vapor deposition method. Xu et al. 2006 suggested the bound magnetic polaron model for long-range ferromagnetic coupling between Mn2+–Mn2+ in Mn and N co-doped ZnO thin films. The observed enhancement in magnetism is due to the hybridization between N 2p and Cr 3d states, which is strong enough to produce hole-mediated ferromagnetism at room temperature. The ferromagnetic coupling weakly depends on the direction of aligned Cr ions, while strongly depends on the distance of N from Cr. So observed enhancement in magnetism is in agreement with Zener’s model for hole-mediated ferromagnetism in wide band gap semiconductors. However, magnetic mechanisms in DMS are still under debate. Additional work is required using X-ray absorption spectroscopy to explain the fundamental physical processes.
Conclusions
In the present study, Cr and N co-doped ZnO nanoparticles have been synthesized successfully through sol–gel technique. Structural analysis indicated that the pure ZnO, Cr-doped ZnO as well as N and Cr co-doped ZnO nanoparticles were hexagonal in structure (wurtzite phase) and no other secondary phase was found in all the samples indicating that Cr cations are substituted at the Zn sites and N replace the O sites. TEM results are in agreement with the size estimated from Scherrer’s formula. The M–H curves at room temperature indicate the enhancement in magnetism with N co-doping in Cr-doped ZnO nanoparticles. The present studies demonstrate that a simple method can be used to incorporate Cr and N into nanocrystalline ZnO for making DMS at room temperature. These nanocrystalline powders may potentially be useful for spintronics applications.
References
Assadi MHN, Zhang YB, Li S (2009) Substantial stabilization of ferromagnetism in ZnO:Mn induced by N codoping. J Phys: Condens Matter 21:185503
Coey JMD, Venkatesan M, Fitzgerald CB (2005) Donor impurity band exchange in dilute ferromagnetic oxides. Nat Mater 4:173
Deka S, Pasricha R, Joy PA (2004) Synthesis and ferromagnetic properties of lightly doped noncrystalline Zn 1-x Co x O. Chem Mater 16:1168
Dietl T, Ohno H, Matsukura F, Cibert J, Ferrand D (2000) Zener model description of ferromagnetism in Zinc-Blende magnetic semiconductors. Science 287:1019
Duan LB, Zhao XR, Liu JM, Wang T, Rao GH (2010) Room-temperature ferromagnetism in lightly Cr-doped ZnO nanoparticles. Appl Phys A 99:679
Furdyna JK (1988) Diluted magnetic semiconductors. J Appl Phys 64:R29
Kaminska E, Piotrowska A, Kossut J, Barcz A, Butkute R, Dobrowolski W, Dynowska E, Jakiela R, Przezdziecka E, Lukasiewicz R, Aleszkiewicz M, Wojnar P, Kowalczyk E (2005) Transparent p-type ZnO films obtained by oxidation of sputter-deposited Zn3N2. Solid State Commun 135:11
Katayama-Yoshida H, Sato K, Fukushima T, Toyoda M, Kizaki H, Dinh VA, Dederichs PH (2007) Theory of ferromagnetic semiconductors. Phys. Stat. Sol. (A) 204:15
Kittilstved KR, Liu WK, Gamelin DR (2006) Elecronic structure oigins of polarity dependent high Tc ferromagnetism in oxide-diluted magnetic semiconductors. Nat Mater 5:291–297
Kittistved KR, Norberg NS, Gamelin DR (2005) Chemical manipulation of high-Tc ferromagnetism in ZnO diluted magnetic semiconductors. Phys Rev Lett 94:147209
Lee Y, Lee JC, Su CW (2010) Effects of N-doping on magnetic properties of ZnCoO diluted magnetic semiconductor thin films. IEEE Trans Magn 46:1565
Li L, Wang W, Liu H, Liu X, Song Q, Ren S (2009) First principles calculations of electronic band structure and optical properties of Cr-doped ZnO. J Phys Chem C 113:8460
Liu H, Zhang X, Li L, Wang YX, Gao KH, Li ZQ, Zheng RK, Ringer SP, Zhang B, Zhang XX (2007) Role of point defects in room-temperature ferromagnetism of Cr-doped ZnO. Appl Phys Lett 91:072511
McNorton RD, MacLaren JM (2009) A study of the disordered dilute magnetic semiconductors Zn1-x Crx Se and Zn1-x Fex Se. J Phys: Condens Matter 21:445803
Ohno H (1998) Making non-magnetic semiconductors ferromagnetic. Science 281:951
Pandiyarajan T, Karthikeyan B (2012) Cr-doping induced structural, phonon and excitonic properties of ZnO nanoparticles. J Nanopart Res 14:647
Pearton SJ, Abernathy CR, Overberg ME, Thaler GT, Norton DP, Theodoropoulou N, Hebard AF, Park YD, Ren F, Kim J, Boatner LA (2003) Wide band gap semiconductors and oxides. J Appl Phys 93:1
Prellier W, Fouchet A, Mercey B, Simon C, Raveau B (2003) Laser ablation of Co:ZnO films deposited from Zn and Co metal targets on (0001) Al2O3 substrates. Appl Phys Lett 82:3490
Roberts BK, Pakhomov AB, Shutthanandan VS, Krishnan KM (2005) Ferromagnetic Cr-doped ZnO for spin electronics via magneton sputtering. J Appl Phys 97:10D310
Sato K, Katayama-Yoshida H (2000) Material design for transparent ferromagnets with ZnO-based magnetic semiconductors. Jpn J Appl Phys 39:L555
Sato K, Katayama-Yoshida H (2001) Stabilization of ferromagnetic states by electron doping in Fe, Co or Ni-doped ZnO. Jpn J Appl Phys 40:L334
Schneider L, Zaitsev SV, Jin W, Kompch A, Winterer M, Acet M, Bacher G (2009) Fabrication and analysis of Cr-doped ZnO nanoparticles from the gas phase. Nanotechnology 20:135604
Sernelius BE, Berggren KF, Jin ZC, Hamberg I, Granqvist CG (1988) Band-gap tailoring of ZnO by means of heavy Al doping. Phys Rev B 37:10244
Sundaresan A, Bhargavi R, Rangarajan N, Siddesh U, Rao CNR (2006) Ferromagnetism is a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys Rev 74:161306 R
Tampo H, Shibata H, Maejima K, Yamada A, Matsubara K, Fons P, Niki S, Tainaka T, Chiba Y, Kanie H (2007) Strong excitonic transition of Zn1-xMgxO alloy. Appl Phys Lett 91:261907
Ueda K, Tabata H, Kawai T (2001) Magnetic and electric properties of transition-metal-doped ZnO films. Appl Phys Lett 79:988
Van Dijken A, Meulenkamp EA, Vanmaekelbergh D, Meijerink A (2000) Identification of the transition responsible for the visible emission in ZnO using quantum size effects. J Lumin 90:123
Wolf SA, Awschalom DD, Buhrman RA, Daughton JM, Molnar SV, Roukes ML, Chtchelkanova AY, Treger DM (2001) Spintronics: a spin based electronics vision for the future. Science 294:1488
Xu HY, Liu YC, Xu CS, Liu YX, Shao CL, Mu R (2006) Room-temperature ferromagnetism in (Mn, N)- Co doped ZnO thin films prepared by reactive magnetron cosputtering. Appl Phys Lett 88:242502
Yan W, Sun Z, Liu Q, Li Z, Shi T, Wang F, Qi Z, Zhang G, Zhang SWH, Chen Z (2007) Structures and magnetic properties of (Mn, N)-codoped ZnO thin films. Appl Phys Lett 90:242509
Zhang SB, Wei SH, Zunger A (2001) Intrinsic n-type versus p-type doping asymmetry and the defect physics of ZnO. Phys Rev B 63:075205
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kaur, P., Kumar, S., Negi, N.S. et al. Enhanced magnetism in Cr-doped ZnO nanoparticles with nitrogen co-doping synthesized using sol–gel technique. Appl Nanosci 5, 367–372 (2015). https://doi.org/10.1007/s13204-014-0326-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0326-1