Abstract
The development of eco-friendly alternative to chemical synthesis of metal nanoparticles is of great challenge among researchers. The present study aimed to investigate the biological synthesis, characterization, antimicrobial study and synergistic effect of silver and zinc oxide nanoparticles against clinical pathogens using Pichia fermentans JA2. The extracellular biosynthesis of silver and zinc oxide nanoparticles was investigated using Pichia fermentans JA2 isolated from spoiled fruit pulp bought in Vellore local market. The crystalline and stable metallic nanoparticles were characterized evolving several analytical techniques including UV–visible spectrophotometer, X-ray diffraction pattern analysis and FE-scanning electron microscope with EDX-analysis. The biosynthesized metallic nanoparticles were tested for their antimicrobial property against medically important Gram positive, Gram negative and fungal pathogenic microorganisms. Furthermore, the biosynthesized nanoparticles were also evaluated for their increased antimicrobial activities with various commercially available antibiotics against clinical pathogens. The biosynthesized silver nanoparticles inhibited most of the Gram negative clinical pathogens, whereas zinc oxide nanoparticles were able to inhibit only Pseudomonas aeruginosa. The combined effect of standard antibiotic disc and biosynthesized metallic nanoparticles enhanced the inhibitory effect against clinical pathogens. The biological synthesis of silver and zinc oxide nanoparticles is a novel and cost-effective approach over harmful chemical synthesis techniques. The metallic nanoparticles synthesized using Pichia fermentans JA2 possess potent inhibitory effect that offers valuable contribution to pharmaceutical associations.
Similar content being viewed by others
Introduction
In the modern science of nonmaterial, the interaction between inorganic nanoparticles and biological structures is one of the most emerging and exciting areas of research. The research in nanobiotechnology deals with the development of eco-friendly processes for the synthesis of stable nanoparticles, possessing well-defined shapes and controlled narrow sizes (Kathiresan et al. 2009). Nanoparticles have been abundantly used in nanochemistry to enhance the immobilization and activity of catalysts (Wang 2006) in pharmaceutical nanoengineering for delivery of therapeutic agents (Zhang et al. 2008) in chronic disease diagnostics and in sensors (Hong et al. 2008). Silver nanoparticles (Ag NPs) and zinc oxide nanoparticles (ZnO NPs) have received considerable attention due to their good conductivity, chemical stability, catalytic property, photonics and optoelectronics, unique antibacterial, antifungal and UV filtering properties (Meruvu et al. 2011). ZnO nanomaterials are also being considered for use in next-generation biological applications including antimicrobial agents, drug delivery and bioimaging probes (Padmavathy and Vijayaraghavan 2008). The ever increasing antibiotic resistance in pathogenic and opportunistic microorganisms encourages the scientific community to constantly develop new drugs and antimicrobial agents. In the last decade, several new antibiotics have been introduced by pharmaceutical industries and none of them has improved the activity against multi-drug resistant bacteria (Conlon et al. 2004). Metallic nanoparticles have demonstrated antimicrobial activities to the development of novel applications in this field, making them an attractive alternative to antibiotics (Fidel et al. 2010). With the prevalence and increase of microorganisms resistant to multiple antibiotics and the continuing emphasis on health care costs, many researchers have tried to develop new, effective antimicrobial agents, free of resistance and cost-effective. To avoid the use of toxic organic solvents and severe reaction conditions (temperature, pressure and long refluxing time) for the preparation of nanomaterials, researchers recently have been exploring the possibilities of preparing nanomaterials in aqueous medium with the help of stabilizing or capping agents (Shervani and Yamamoto 2011). In this work, we report extracellular biosynthesis of silver (Ag) and zinc oxide (ZnO) nanoparticles (NPs) from extracellular components of Pichia fermentans JA2. The biosynthesized Ag and ZnO nanoparticles were extensively characterized through sophisticated analytical instrumentation and their antimicrobial activity was evaluated against various pathogenic bacteria and fungi.
Experimental
Materials and methods
Sample collection
Spoiled fruits were collected from local fruit market in the month of January 2011 from Vellore, Tamil Nadu, India.
Isolation of Pichia fermentans JA2
The isolation of Pichia fermentans JA2 was carried out by standard serial dilution method and spread plate was performed on malt yeast peptone glucose agar (yeast extract 0.3 %, malt extract 0.3 %, peptone 0.5 %, glucose 1 % and agar 1.5 %). The colonies appeared were further purified by repeatedly streaking on malt yeast peptone glucose agar (MYPGA).
Molecular identification of isolated yeast
The extraction of genomic DNA of the strain was performed according to the method described by Rainey et al. (1996). 18S rRNA gene was amplified with the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The amplified DNA fragment was separated on 1 % agarose gel. The purified PCR product was sequenced using the Big-Dye terminator kit ABI 310 Genetic Analyzer (Applied Biosystems, USA). The phylogenetic position of the isolated strain (Pichia fermentans JA2) was assessed by performing a nucleotide sequence database search using the BLAST program from NCBI GenBank. Sequence data of related species were retrieved from NCBI GenBank. The nucleotide sequencing result was submitted to the GenBank National Centre for Biotechnology Information (NCBI) and accession number obtained is KC509579.
Microbial synthesis of Ag NPs
The Pichia fermentans JA2 strain was isolated from the pulp of spoiled fruits. For the synthesis of silver nanoparticles, the active Pichia fermentans JA2 culture was freshly inoculated on sterile potato dextrose broth and the flasks were incubated at 28 °C and 200 rpm for 3 days (Gajbhiye et al. 2009). After the incubation period was complete, the culture was centrifuged (5,000 × g) for 30 min and the supernatant was used for the biosynthesis of Ag NPs. Deionized water was used as a solvent in the synthesis of Ag NPs. The collected supernatant (1 %) was added to conical flask containing 1 mM AgNO3, was further incubated for 96 h at 28 °C and 200 rpm.
Microbial synthesis of ZnO NPs
The Pichia fermentans JA2 was allowed to grow as culture suspension in yeast peptone glucose medium for 24 h. After incubation period was complete, the culture was centrifuged (5,000 × g) for 30 min and supernatant was used for the biosynthesis of ZnO NPs. 0.1 g of zinc oxide was added to 250 ml of Erlenmeyer flasks containing deionized water followed by 1 % Pichia fermentans JA2 culture supernatant (Prasad and Jha 2009; Kirthi et al. 2011). The flask was further incubated at 37 °C under agitation (200 rpm) for 24–48 h.
UV–visible spectroscopy analysis
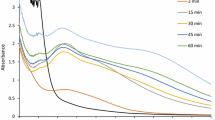
The metal ion reduction was monitored by measuring UV-spectrum of AgNO3 and ZnO treated supernatant periodically. The aliquots of this solution were monitored for UV-spectra after 1, 24 and 48 h. The UV–Vis spectroscopy measurements were recorded on HITACHI, Model U-2800 spectrophotometer from 300 to 600 nm. The Ag NPs and ZnO NPs dispersed in deionized water were observed for their surface plasmon resonance at 420 and 374 nm, respectively (Jayaseelan et al. 2012). The supernatant of AgNO3 and ZnO was used separately as control 1 and 2, respectively.
X-ray diffraction pattern analysis
The biosynthesized Ag NPs and ZnO NPs were freeze-dried and powdered in order to analyze XRD pattern (Sadhasivam et al. 2010). The phase formation and purity of metallic nanoparticles were checked through XRD patterns which were recorded using powder X-ray diffractometer (Model-D8 Advance, made in BRUKER Germany).
Electronic microscopic study
Biosynthesized Ag NPs and ZnO NPs were mounted on specimen stubs with double-sided adhesive tape coated with gold in a sputter coater and examined under field emission scanning electron microscopy (HITACH, Model E-1010 Ion sputter) to avoid charging and examined under SEM (HITACH, Model S-3400 N). The presence of silver metal ions in the sample was confirmed by energy dispersive X-ray analysis (EDXA) combined with field emission scanning electron microscopy.
Antimicrobial activity of Ag NPs
The antimicrobial activity of extracellularly biosynthesized Ag NPs and ZnO NPs against pathogenic organisms such as Gram positive (Enterococcus sp. and Staphylococcus aureus), Gram negative (Escherichia coli, Salmonella sp., Shigella sp., Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa) and fungal strains (Candida tropicalis, Fusarium sp., Scedosporium sp. JAS1, Ganoderma sp. JAS4, Aspergillus terreus strain JAS1) was measured using well-diffusion method (Jeevan et al. 2012). The bacterial test organisms were acquired from Microbial Biotechnology Lab, SBST, VIT University, Vellore, India.
Antibiogram of clinical pathogens
The multi-drug resistant bacterial test pathogens were screened against standard antibiotic disc, vancomycin (30 mcg/disc), tigecycline (15 mcg/disc), erythromycin (15 mcg/disc), ciprofloxacin (30 mcg/disc), penicillin (10 mcg/disc), ofloxacin (5 mcg/disc), and fungal isolates were screened against fluconazole (25 mcg/disc) and voriconazole (5 mcg/disc). Antibiotic sensitivity test was performed by Kirby–Bauer method on Muller–Hinton agar plates. Bacterial test pathogens were lawn cultured on Muller–Hinton agar plates using sterile cotton swabs. The standard antibiotic discs were placed on it using sterile forceps. The plates were incubated at 37 °C for 24–48 h and were observed for zone of inhibition. Fungal test pathogens were seeded into potato dextrose agar petridishes, antibiotic disc was placed on it using sterile forceps. Plates were incubated at 30 °C for 5 days and were observed for zone of inhibition.
Synergistic effect of Ag NPs and ZnO NPs
A disc diffusion method was used to assay the synergistic effect of antibiotics with extracellularly synthesized Ag NPs and ZnO NPs for bactericidal activity against test strains on Muller–Hinton agar plates. To determine the synergistic effect, each standard antibiotic disc was further impregnated with 10 μl of freshly prepared Ag NPs and ZnO NPs. Muller–Hinton agar medium plates were seeded with 100 μl of test organisms, and antibiotic disc impregnated with Ag NPs and ZnO NPs was placed onto agar plates (Fayaz et al. 2010). After incubation at 37 °C for 24–48 h, the zones of inhibition were measured.
Results and discussion
Isolation and characterization
The pure colonies were obtained and the isolate was characterized as Pichia fermentans JA2 based on the molecular characterization through 18S rRNA sequencing studies.
Microbial synthesis of Ag NPs and ZnO NPs
The silver nanoparticles were successfully synthesized by the culture supernatant of Pichia fermentans JA2. The appearance of a yellowish-brown color in the silver nitrate treated flask indicated the formation of silver nanoparticles, whereas no color change was observed in either the culture supernatant without silver nitrate. Pichia fermentans JA2 culture exhibited noticeable white clusters deposited at the bottom of flask indicating the formation of ZnO NPs in the medium after 72 h of incubation.
UV–visible spectrophotometer
The formation and stability of the reduced metallic nanoparticles in the colloidal solution were monitored by using UV absorption spectra. The UV–visible spectrum from Pichia fermentans JA2 reaction vessel at different time of reaction is plotted in Figs. 1 and 2 indicating the presence of Ag NPs and ZnO NPs, respectively. A strong and broad peak was observed between 420 and 425 nm confirming the formation of Ag NPs which is similar to the findings of Kumar et al. (2011). An absorption peak observed at 374 nm indicates the successful biosynthesis of ZnO NPs which is in good agreement with previously reported work by Jayaseelan et al. (2012).
It has been proposed that increase in the intensity is due to the excitation of surface plasmon resonance (SPR) and the synthesis of metal nanoparticles depends on the nitrate reductase enzyme present in the microbes. The mechanism of the biosynthesized nanoparticles involves the reduction of silver ions by the electron shuttle enzymatic metal reduction process (Sadhasivam et al. 2010). The mechanism of the biosynthesized nanoparticles involves the reduction of silver ions by the electron shuttle enzymatic metal reduction process. Two enzymes including NADH and NADH-dependent enzymes are important factors in the biosynthesis of metal nanoparticles. Microbes secrete known enzymes such as cofactor NADH, and NADH-dependent enzymes like nitrate reductase might be responsible for the bioreduction of metal ions and the subsequent formation of silver nanoparticles (Kalimuthu et al. 2008).
X-ray diffraction pattern
The XRD pattern of silver nitrate treated sample shows four intense peaks in whole spectrum 2θ ranging from 10 to 90. The characteristic XRD peaks were centered at 38°, 45° and 65°, which could be induced by the following crystalline planes of silver (111), (200) and (220), respectively. XRD analysis showed the diffraction peaks at which the indexed planes were about 111, 200 and 220 of the cubic face-centered silver. The strong and narrow diffraction peaks indicate that the product has well crystalline structure as shown in Figs. 3 and 4. The XRD peaks at 31°, 34°, 47°, 56°, 66° and 75° were identified as (100), (002), (101), (102), (110), (112) and (202) reflections, respectively.
FE–SEM–EDX analysis
The purity of the biosynthesized Ag NPs and ZnO NPs was examined by EDXA combined with FE–SEM. FE–SEM images were measured and topographical analysis was performed based upon the surface study. The biosynthesized Ag NPs which are smooth and rectangular in shape, and energy dispersive spectroscopic analysis of extracellularly synthesized Ag NPs are shown in Figs. 5 and 6, respectively. It has been demonstrated that the size, shape, surface area, solubility, chemical composition and dispersion factor of NPs play exceptional roles in determining their biological responses (Oberdorster et al. 2005). ZnO NPs are also smooth and elongated in shape and their EDXA spectrum revealed a strong signal for silver as well zinc oxide nanoparticles as shown in Figs. 7 and 8 which further confirms the biosynthesis of metal nanoparticles.
Antimicrobial activity of biosynthesized nanoparticles
The antimicrobial activity of extracellular biosynthesized Ag NPs and ZnO NPs was evaluated against various pathogenic bacteria and fungi by Kirby–Bauer method. Escherichia coli, Pseudomonas aeruginosa, Salmonella sp., Staphylococcus aureus, Aspergillus tereus strain JAS1, Scedosporium sp. JAS1, Candida tropicalis and Fusarium sp. were effectively inhibited by biosynthesized Ag NPs as shown in Tables 1 and 2. The strong inhibitory activity of ZnO NPs was manifested against Pseudomonas aeruginosa and Fusarium sp. as presented in Tables 3 and 4.
Antibiogram studies
The clinical pathogens exhibited multiple drug resistance to various antibiotics. All the bacterial pathogens showed resistance toward vancomycin (30 mcg/disc) followed by tetracycline (15 mcg/disc) and erythromycin (15 mcg/disc). Ofloxacin (5 mcg/disc) was found to be sensitive against all the bacteria clinical pathogens as shown in Table 5. The antibiotic sensitivity test for fungal strains is presented in Table 6.
Synergistic effect of metallic nanoparticles
The synergistic approach attempts the combination of antibiotics with biosynthesized metallic nanoparticles against clinical pathogens and offers valuable contribution to nanomedicine. The antibacterial activity of tigecycline, vancomycin, erythromycin and ofloxacin increased in the presence of synthesized metallic nanoparticles against test strains as shown in Tables 7 and 8. The increase in synergistic effect may be caused by the bonding reaction between antibiotic and nanomaterials. The antibiotic molecules contain many active groups such as hydroxyl and amide groups, which react easily with nanosilver by chelation. The combined effect of Ag NPs synthesized nanoparticles and antibiotics was promising against Klebsiella pneumoniae followed by Salmonella sp., Proteus mirabilis and in case of ZnO NPs, Enterococcus sp., Staphylococcus aureus, Proteus mirabilis showed effective zone of inhibition. The biosynthetic methods have been recognized as an alternative to chemical and physical synthesis as biosynthetic method is economical, ecofriendly and green low cost approach. In this study, Ag NPs and ZnO NPs were synthesized by extracellular components of Pichia fermentans JA2. The biosynthetic route developed in this study for producing metallic nanoparticles has distinct advantages over chemical synthetic techniques regarding biosafety and offers valuable contribution to pharmaceutical associations.
References
Conlon JM, Kolodziejek J, Nowotny N (2004) Antimicrobial peptides from ranid frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim Biophys Acta 1696:1–14
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R (2010) Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against Gram-positive and Gram-negative bacteria. Nanomed Nanotechnol 6:103–109
Fidel MG, Peggy L, Adriana B, Erasmo O, Nereyda N, Elpidio MS, Facundo R, Horacio B, Yossef AG (2010) Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomed Nanotechnol 6:681–688
Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M (2009) Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed Nanotechnol 5:382–386
Hong B, Kai J, Ren Y, Han J, Zou Z, Ahn CH (2008) Highly sensitive rapid, reliable, and automatic cardiovascular disease diagnosis with nanoparticle fluorescence enhancer and MEMS. Adv Exp Med Biol 614:265–273
Jayaseelan C, Rahuman A, Kirthi A, Marimuthu S, Santhoshkumar T, Bagavan A, Gaurav K, Karthik L, Rao K (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta A 90:78–84
Jeevan P, Ramya K, Reena A (2012) Extracellular biosynthesis of silver nanoparticles by culture supernatant of Pseudomonas aeruginosa Indian. J Biotechnol 11:72–76
Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S (2008) Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Bioint 65:150–153
Kathiresan K, Manivannan S, Nabeel MA, Dhivya B (2009) Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B 71:133–137
Kirthi AV, Rahuman AA, Marimuthu S, Santhoshkumar T, Jayaseelan C, Kanayairam V (2011) Acaricidal, pediculocidal and larvicidal activity of synthesized ZnO nanoparticles using wet chemical route against blood feeding parasites. Parasitol Res 109:461–472
Kumar A, Pandey AK, Singh SS, Shankar R, Dhawan A (2011) Cellular uptake and mutagenic potential of metal oxide nanoparticles in bacterial cells. Chemosphere 83:1124–1132
Meruvu H, Vangalapati M, Chippada SC, Bammidi SR (2011) Synthesis and characterization of zinc oxide nanoparticles and its antimicrobial activity against Bacillus subtilis and Escherichia coli. J Rasayan Chem 4:217–222
Oberdorster G, Maynard A, Donaldson K, Castranova V (2005) Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol 2:8
Padmavathy N, Vijayaraghavan R (2008) Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study. Sci Technol Adv Mater 9:7
Prasad K, Jha AK (2009) ZnO nanoparticles and adsorption study. Nat Sci 1:129–135
Rainey FA, Rainey NW, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsiaceae fam. nov. Int J Syst Bacteriol 46:1088–1092
Sadhasivam S, Shanmugam P, Yun K (2010) Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B 81:358–362
Shervani Z, Yamamoto Y (2011) Size and morphology controlled synthesis of gold nanoparticles in green solvent. Mater Lett 65:92–95
Wang P (2006) Nanoscale biocatalyst systems. Curr Opin Biotechnol 17:574–579
Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83:761–769
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Chauhan, R., Reddy, A. & Abraham, J. Biosynthesis of silver and zinc oxide nanoparticles using Pichia fermentans JA2 and their antimicrobial property. Appl Nanosci 5, 63–71 (2015). https://doi.org/10.1007/s13204-014-0292-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0292-7